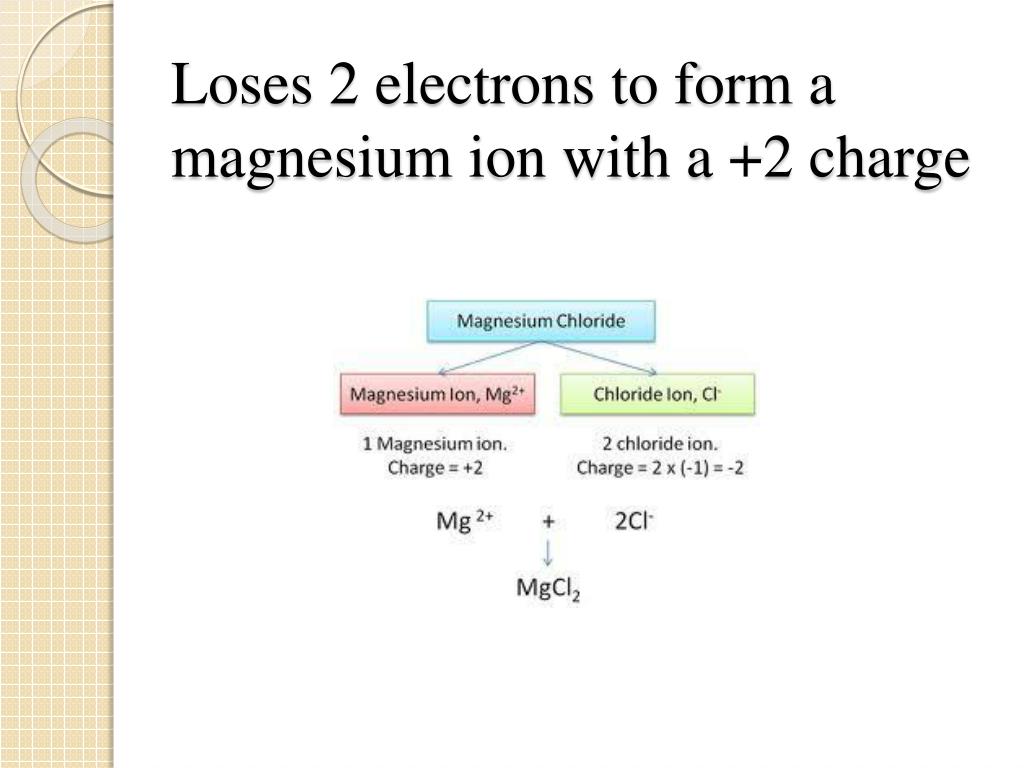
Magnesium Ion Formula And Charge . The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Write formulas for ionic compounds. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound. Magnesium and bromine are used as an example. Predict the charge of monatomic main group elements based on their group number. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−.
from www.slideserve.com
Write formulas for ionic compounds. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict the charge of monatomic main group elements based on their group number. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the symbol for each ion and name them. Magnesium and bromine are used as an example. Magnesium and nitrogen react to form an ionic compound. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative.
PPT Quiz Review PowerPoint Presentation, free download ID2732026
Magnesium Ion Formula And Charge The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Magnesium and nitrogen react to form an ionic compound. Write formulas for ionic compounds. Predict the charge of monatomic main group elements based on their group number. Magnesium and bromine are used as an example.
From slideplayer.com
Naming Ionic Compounds. ppt download Magnesium Ion Formula And Charge Magnesium and bromine are used as an example. Magnesium and nitrogen react to form an ionic compound. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Predict which forms an anion, which forms a cation, and the charges of each ion. Learn how to figure. Magnesium Ion Formula And Charge.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Ion Formula And Charge Write the symbol for each ion and name them. Predict which forms an anion, which forms a cation, and the charges of each ion. Write formulas for ionic compounds. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium and nitrogen react to form an. Magnesium Ion Formula And Charge.
From www.pw.live
Magnesium Iodide Formula, Structure And Properties Magnesium Ion Formula And Charge Write formulas for ionic compounds. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The. Magnesium Ion Formula And Charge.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Magnesium Ion Formula And Charge Magnesium and nitrogen react to form an ionic compound. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom. Magnesium Ion Formula And Charge.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion Formula And Charge When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict which forms an anion, which forms a cation, and the charges of each ion. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Magnesium Ion Formula And Charge.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Formula And Charge Write formulas for ionic compounds. Magnesium and bromine are used as an example. Write the symbol for each ion and name them. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Predict which forms an anion, which forms a cation, and the charges of each. Magnesium Ion Formula And Charge.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Ion Formula And Charge Magnesium and bromine are used as an example. Predict which forms an anion, which forms a cation, and the charges of each ion. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has. Magnesium Ion Formula And Charge.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Magnesium Ion Formula And Charge When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict which forms an anion, which forms a cation, and the charges of each ion. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Magnesium Ion Formula And Charge.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Ion Formula And Charge The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Magnesium and bromine are used as an example. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Predict the charge of monatomic main group elements based on their group number.. Magnesium Ion Formula And Charge.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Formula And Charge Magnesium and nitrogen react to form an ionic compound. Write formulas for ionic compounds. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Learn how to figure out the formulas. Magnesium Ion Formula And Charge.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Ion Formula And Charge Magnesium and bromine are used as an example. Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which. Magnesium Ion Formula And Charge.
From www.youtube.com
magnesium ion charge YouTube Magnesium Ion Formula And Charge When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and name them. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely. Magnesium Ion Formula And Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Ion Formula And Charge The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Predict the charge of monatomic main group elements based on their group. Magnesium Ion Formula And Charge.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Magnesium Ion Formula And Charge Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus. Magnesium Ion Formula And Charge.
From www.youtube.com
Draw the Lewis Structure of MgI2 (magnesium iodide) YouTube Magnesium Ion Formula And Charge Magnesium and bromine are used as an example. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. When an ionic compound is formed from magnesium and oxygen, the magnesium ion. Magnesium Ion Formula And Charge.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ion Formula And Charge Write the symbol for each ion and name them. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Predict the charge of monatomic main group elements based on their group number. The formula of an ionic compound represents the simplest ratio of the numbers of. Magnesium Ion Formula And Charge.
From www.slideserve.com
PPT Lesson Objectives Write formulas for Common polyatomic ions Ionic Magnesium Ion Formula And Charge Predict the charge of monatomic main group elements based on their group number. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the symbol for each ion and name them. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion,. Magnesium Ion Formula And Charge.
From slidetodoc.com
Metal ions Nonmetal ions Positive ion Gain electrons Magnesium Ion Formula And Charge Write formulas for ionic compounds. Predict the charge of monatomic main group elements based on their group number. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely. Magnesium Ion Formula And Charge.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Formula And Charge Predict which forms an anion, which forms a cation, and the charges of each ion. Write formulas for ionic compounds. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Magnesium and bromine are used as an example. The element magnesium is found in group two of the periodic table, so a magnesium ion is. Magnesium Ion Formula And Charge.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Formula And Charge Predict which forms an anion, which forms a cation, and the charges of each ion. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Write the symbol for each ion and name them. Write formulas for ionic compounds. Magnesium and bromine are used as an. Magnesium Ion Formula And Charge.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion Formula And Charge The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Magnesium and bromine are used as an example. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them. Learn how to figure. Magnesium Ion Formula And Charge.
From ar.inspiredpencil.com
Magnesium Ion Lewis Dot Structure Magnesium Ion Formula And Charge Predict which forms an anion, which forms a cation, and the charges of each ion. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Predict the charge of monatomic main group elements based on their group number. The element magnesium is found in group two. Magnesium Ion Formula And Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Ion Formula And Charge When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the symbol for each ion and name them. Magnesium and bromine are used as an example. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict the charge of. Magnesium Ion Formula And Charge.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Magnesium Ion Formula And Charge Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Write the symbol for each ion and name them. When an ionic. Magnesium Ion Formula And Charge.
From schematicfixlankier.z21.web.core.windows.net
Bohr Diagram For Magnesium Magnesium Ion Formula And Charge The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Magnesium and bromine are used as an example. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Write the symbol for each ion and name them. Predict the charge of. Magnesium Ion Formula And Charge.
From www.slideserve.com
PPT Quiz Review PowerPoint Presentation, free download ID2732026 Magnesium Ion Formula And Charge Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the symbol for each ion and name them. Write formulas for. Magnesium Ion Formula And Charge.
From www.science-revision.co.uk
Protons, neutrons and electrons. Magnesium Ion Formula And Charge Write formulas for ionic compounds. Predict which forms an anion, which forms a cation, and the charges of each ion. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Magnesium and bromine are used as an example. The element magnesium is found in group two of the periodic table, so a magnesium ion is. Magnesium Ion Formula And Charge.
From www.slideshare.net
Bonding Basics Magnesium Ion Formula And Charge Write the symbol for each ion and name them. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Predict the charge. Magnesium Ion Formula And Charge.
From slideplayer.com
Chemistry Chapter 9 Chemical Formulas and Chemical Compounds ppt download Magnesium Ion Formula And Charge The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Predict the charge of monatomic main group elements based on their group number. Magnesium and bromine are used as an example. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a. Magnesium Ion Formula And Charge.
From slideplayer.com
Ionic charge and electrical conduction ppt download Magnesium Ion Formula And Charge Write formulas for ionic compounds. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. The element magnesium is found in group. Magnesium Ion Formula And Charge.
From www.slideshare.net
Bonding Basics Magnesium Ion Formula And Charge Magnesium and bromine are used as an example. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write formulas for ionic compounds. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. The formula of an ionic compound represents the. Magnesium Ion Formula And Charge.
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium Ion Formula And Charge Magnesium and nitrogen react to form an ionic compound. Magnesium and bromine are used as an example. Predict which forms an anion, which forms a cation, and the charges of each ion. The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Write formulas for ionic. Magnesium Ion Formula And Charge.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Ion Formula And Charge Write formulas for ionic compounds. Predict which forms an anion, which forms a cation, and the charges of each ion. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2−. Write the symbol for each ion and name them. The formula of an ionic compound represents. Magnesium Ion Formula And Charge.
From www.slideshare.net
Covalent and ionic review do this first!!! Magnesium Ion Formula And Charge Predict the charge of monatomic main group elements based on their group number. Magnesium and nitrogen react to form an ionic compound. The formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of positive and negative. Magnesium and bromine are used as an example. The element magnesium is found in. Magnesium Ion Formula And Charge.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Ion Formula And Charge The element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Write the symbol for each ion and name them. Learn how to figure out the formulas of ionic compounds, including the handy crisscross rule. Magnesium and nitrogen react to form an ionic compound. The formula of. Magnesium Ion Formula And Charge.