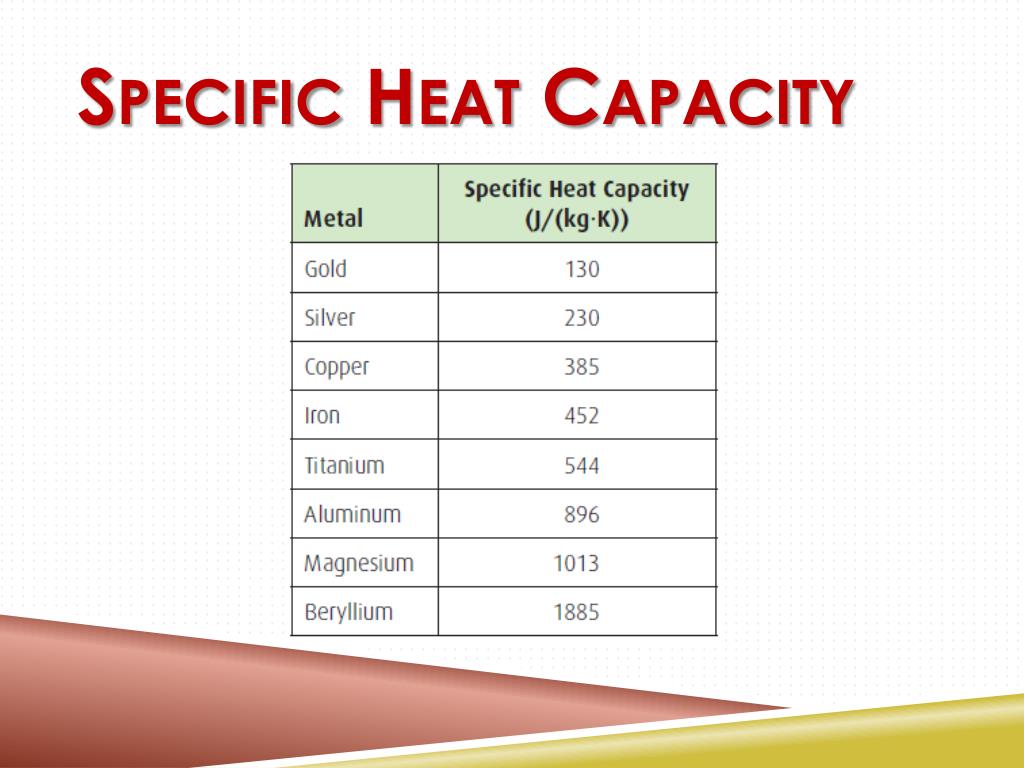
What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab . the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Δtδt will determine the sign of the. the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). The most efficient insulation (apart from a. Where is heat in joules, is the specific. in this experiment, you will determine the specific heat capacities of two different unknown metals by. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. now we will examine the relationship between heat and specific heat capacity: you can find specific heat capacity for different substances in table 5.2.1 on this page. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the.
from www.slideserve.com
The most efficient insulation (apart from a. the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). now we will examine the relationship between heat and specific heat capacity: in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. in this experiment, you will determine the specific heat capacities of two different unknown metals by. Δtδt will determine the sign of the. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Where is heat in joules, is the specific. you can find specific heat capacity for different substances in table 5.2.1 on this page.
PPT Specific Heat Capacity PowerPoint Presentation, free download
What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. Where is heat in joules, is the specific. The most efficient insulation (apart from a. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Δtδt will determine the sign of the. in this experiment, you will determine the specific heat capacities of two different unknown metals by. you can find specific heat capacity for different substances in table 5.2.1 on this page. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. now we will examine the relationship between heat and specific heat capacity: the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”).
From www.studypool.com
SOLUTION CHEM 3H1 USTP Calorimeter Specific Heat Capacity Lab Report What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. now we will examine the relationship between heat and specific heat capacity: the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). the. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.studypool.com
SOLUTION Physics specific heat capacity lab Studypool What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. you can find specific heat capacity for different substances in table 5.2.1 on this page. now we will examine the relationship between heat and specific heat capacity: in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. the heat capacity of. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. The most efficient insulation (apart from a. you can find specific heat capacity for different substances in table 5.2.1 on this page. — the amount of heat absorbed or released (q) by the object depends on its mass (m),. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.answersarena.com
[Solved] Chapter 3 Calorimetry Read and write up how you What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab in this experiment, you will determine the specific heat capacities of two different unknown metals by. Δtδt will determine the sign of the. you can find specific heat capacity for different substances in table 5.2.1 on this page. The most efficient insulation (apart from a. in this laboratory experiment, we use calorimetry to determine the specific heat. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. in this experiment, you will determine the. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From studylib.net
LAB 3 SPECIFIC HEAT CAPACITY (method of What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab Where is heat in joules, is the specific. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. you can find specific heat capacity for different substances in table 5.2.1 on this page. in this experiment, you will determine the specific heat capacities of. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.studocu.com
Lab 2 Specific Heat Capacity 2 Physics 4C Spring 2023 Lab 2 What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab Δtδt will determine the sign of the. The most efficient insulation (apart from a. the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. . What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From haipernews.com
How To Calculate Heat Capacity Of Gold Haiper What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab Δtδt will determine the sign of the. you can find specific heat capacity for different substances in table 5.2.1 on this page. the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). The most efficient insulation (apart from a. in this laboratory experiment, we use calorimetry to. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab Where is heat in joules, is the specific. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. in this experiment, you will determine the specific heat capacities of two different unknown metals by. in this laboratory experiment, we use calorimetry to determine the specific heat capacity. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab in this experiment, you will determine the specific heat capacities of two different unknown metals by. the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). you can find specific heat capacity for different substances in table 5.2.1 on this page. — the amount of heat. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The most efficient insulation (apart from a. now we will examine the relationship between. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab now we will examine the relationship between heat and specific heat capacity: the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Where is heat in joules, is the specific. in this experiment, you will determine the specific heat capacities of two different unknown metals by. . What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From mavink.com
Formula Of Specific Heat Capacity What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). in this experiment, you will determine the specific heat capacities of two different unknown metals by. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s),. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.studocu.com
Calorimetry Part 1 Specific Heat Capacity Chem 200 Lab Report What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab Where is heat in joules, is the specific. in this experiment, you will determine the specific heat capacities of two different unknown metals by. The most efficient insulation (apart from a. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Δtδt will determine the sign of the.. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. The most efficient insulation (apart from a. now we will examine the relationship between heat and specific heat capacity: Δtδt will determine the sign of the. you can find specific heat capacity for different substances in table 5.2.1 on. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. you can find specific heat capacity for different substances in table 5.2.1 on this page. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. . What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab now we will examine the relationship between heat and specific heat capacity: — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. Δtδt will determine the sign of the. Where is heat in joules, is the specific. you can find specific heat capacity for. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab you can find specific heat capacity for different substances in table 5.2.1 on this page. in this experiment, you will determine the specific heat capacities of two different unknown metals by. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. — the amount of heat. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab now we will examine the relationship between heat and specific heat capacity: in this experiment, you will determine the specific heat capacities of two different unknown metals by. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. — the amount of heat absorbed or released (q) by. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab in this experiment, you will determine the specific heat capacities of two different unknown metals by. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. now we will examine the relationship between heat and specific heat capacity: in this laboratory experiment, we use calorimetry to. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From studymind.co.uk
Heat Capacity Study Mind What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. in this experiment, you will determine the specific heat capacities of two different unknown metals by. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.studypool.com
SOLUTION Specific Heat Capacity Lab Studypool What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). The most efficient insulation (apart from a. you can find specific heat capacity for different substances in table 5.2.1 on this page. now we will examine the relationship between heat and specific heat capacity: in this. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.sliderbase.com
Specific Heat What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. Δtδt will determine the sign of the. the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). the symbol c stands for the specific heat (also called “specific heat. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From slideplayer.com
Including Temperature, Energy, Specific Heat Capacity, and Calorimetry What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. in this experiment, you will determine the specific heat capacities of two different unknown metals by. Δtδt will determine the sign of the. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. in this laboratory experiment, we use calorimetry. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Δtδt will determine the sign of the. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. now we will examine the. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From slidetodoc.com
Specific Heat Capacity The specific heat capacity of What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. the heat capacity of the calorimeter is related to its total mass (a synonym for heat. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From studylib.net
Calorimetry Lab Specific Heat Capacity What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. you can find specific heat capacity for different substances in table 5.2.1 on this page. Where is heat in joules, is the specific. Δtδt will determine the sign of the. now we will examine. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. you can find specific heat capacity for different substances in table 5.2.1 on this page. Where is heat in joules, is the specific. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. in this experiment, you. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From dxoblibct.blob.core.windows.net
Calorimetry Calculations Examples at Miriam Matos blog What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab you can find specific heat capacity for different substances in table 5.2.1 on this page. Δtδt will determine the sign of the. in this experiment, you will determine the specific heat capacities of two different unknown metals by. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. you can find specific heat capacity for different substances in table 5.2.1 on this page. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. Δtδt will determine the sign of the. — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. Where is heat in joules, is the specific. you. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. in this experiment, you will determine the specific heat capacities of two different unknown. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.worksheetsplanet.com
What is Specific Heat What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab — the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. the heat capacity of the calorimeter is related to its total mass (a synonym for heat capacity is “thermal mass”). you can find specific heat capacity for different substances in table 5.2.1 on this. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab The most efficient insulation (apart from a. now we will examine the relationship between heat and specific heat capacity: in this laboratory experiment, we use calorimetry to determine the specific heat capacity of an unknown metal sample. you can find specific heat capacity for different substances in table 5.2.1 on this page. Δtδt will determine the sign. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.
From bitcoinscishow3713.blogspot.com
Specific Heat Capacity Of Gold / Tang 01 Heat Capacity And Calorimetry What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab you can find specific heat capacity for different substances in table 5.2.1 on this page. the symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Where is heat in joules, is the specific. in this laboratory experiment, we use calorimetry to determine the specific heat capacity of. What Is The Actual Specific Heat Capacity Of The Gold In The Calorimetry Lab.