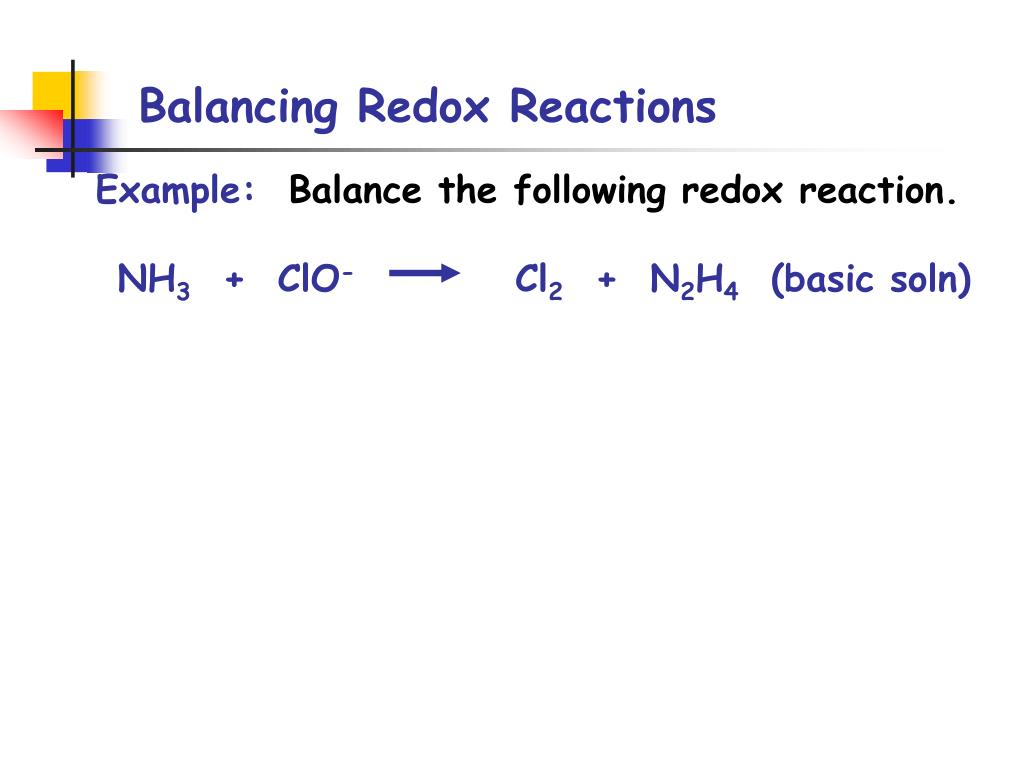
Electrochemistry Balancing Redox Reactions . Assign ons and determine which elements change. Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Understand redox reactions in detail. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts:
from www.slideserve.com
Assign ons and determine which elements change. Understand redox reactions in detail. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Divide the reaction into half reactions using the elements which change as a guide.
PPT Unit 5 Part 2 Redox Reactions and Electrochemistry PowerPoint
Electrochemistry Balancing Redox Reactions To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Understand redox reactions in detail. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Assign ons and determine which elements change.
From www.youtube.com
Electrochemistry Balancing REDOX reactions YouTube Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Understand redox reactions in detail. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Assign ons and determine which elements change. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion. Electrochemistry Balancing Redox Reactions.
From www.youtube.com
Balancing redox reactions in acid Redox reactions and Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Divide the reaction into half reactions using the elements which change as a guide. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Understand redox. Electrochemistry Balancing Redox Reactions.
From www.pinterest.es
Redox Reactions Teaching chemistry, Chemistry classroom, Gcse chemistry Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Assign ons and determine which elements change. Understand redox reactions in detail. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the. Electrochemistry Balancing Redox Reactions.
From www.youtube.com
Balancing Redox Reactions in Acidic and Basic Solution YouTube Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Divide the reaction into half reactions using the elements which change as a guide. Understand redox. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Balancing Redox Reactions PowerPoint Presentation, free download Electrochemistry Balancing Redox Reactions Understand redox reactions in detail. Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Assign ons and determine which elements change. Balancing a redox reaction requires identifying the oxidation numbers in the. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Redox Reactions and Electrochemistry PowerPoint Presentation Electrochemistry Balancing Redox Reactions To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Understand redox reactions in detail. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Assign ons and determine which elements change. Balancing a redox reaction requires identifying. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Balancing Redox Reactions PowerPoint Presentation, free download Electrochemistry Balancing Redox Reactions To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Understand redox reactions in detail. Assign ons and determine which elements change. Divide the reaction into half reactions using the. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Unit 5 Part 2 Redox Reactions and Electrochemistry PowerPoint Electrochemistry Balancing Redox Reactions Assign ons and determine which elements change. Understand redox reactions in detail. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying. Electrochemistry Balancing Redox Reactions.
From www.studypool.com
SOLUTION Chemistry Electrochemistry (Balancing Redox Reactions Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Assign ons and determine which elements change. Understand redox reactions in detail. Divide the reaction into half reactions using the elements which change as a guide. Balancing a redox reaction requires identifying the oxidation numbers in the. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Electrochemistry Balancing Redox Reactions PowerPoint Electrochemistry Balancing Redox Reactions Assign ons and determine which elements change. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Understand redox reactions in detail. To balance a redox. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Electrochemistry Lesson 6 Balancing Redox Reactions PowerPoint Electrochemistry Balancing Redox Reactions Assign ons and determine which elements change. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Divide the reaction into half reactions using the elements which change. Electrochemistry Balancing Redox Reactions.
From www.youtube.com
Electrochemistry 7 Balancing Redox Reactions in Acidic Medium. YouTube Electrochemistry Balancing Redox Reactions To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Assign ons and determine which elements change. Divide the reaction into half reactions using the elements which change. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Electrochemistry Lesson 5 Balancing Half Reactions PowerPoint Electrochemistry Balancing Redox Reactions To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Assign ons and determine which elements change. Understand redox reactions in detail. Balance each half reaction separately, leaving out water,. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3527121 Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Divide the reaction into half reactions using the elements which change as a guide. Understand redox reactions in detail. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into. Electrochemistry Balancing Redox Reactions.
From www.xaktly.com
Redox balancing Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Understand redox reactions in detail. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into. Electrochemistry Balancing Redox Reactions.
From www.youtube.com
Video 3 Balancing Redox Reactions in Basic Solution YouTube Electrochemistry Balancing Redox Reactions Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Assign ons and determine which elements change. Divide the reaction into half reactions using the elements which change as a guide. Understand redox reactions in detail. To balance a redox equation using the oxidation state method, we conceptually separate. Electrochemistry Balancing Redox Reactions.
From chemistry.analia-sanchez.net
Electrochemistry Exercises 2 Balancing redox equations Chemistry Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction. Electrochemistry Balancing Redox Reactions.
From www.scribd.com
15 Electrochemistry Balancing Redox Reactions PDF Redox Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Assign ons. Electrochemistry Balancing Redox Reactions.
From slidetodoc.com
Electrochemistry Redox Oxidation States Voltaic Cells Balancing Equations Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Assign ons and determine which elements change. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the. Electrochemistry Balancing Redox Reactions.
From quizzschoolcartulary.z19.web.core.windows.net
Balance Redox Reaction In Basic Solution Electrochemistry Balancing Redox Reactions Assign ons and determine which elements change. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Understand redox reactions in detail. Divide the reaction into. Electrochemistry Balancing Redox Reactions.
From www.chemistrylearner.com
Redox (OxidationReduction) Reaction Definition & Examples Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Understand redox reactions in detail. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so. Electrochemistry Balancing Redox Reactions.
From studylib.net
Balancing Redox Equations Handout Electrochemistry Balancing Redox Reactions Assign ons and determine which elements change. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Understand redox reactions in detail. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. To balance a redox. Electrochemistry Balancing Redox Reactions.
From slideplayer.com
Electrochemistry Lesson 6 Balancing Redox Reactions. ppt download Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Assign ons and determine which elements change. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction. Electrochemistry Balancing Redox Reactions.
From www.studypool.com
SOLUTION Chemistry Electrochemistry (Balancing Redox Reactions Electrochemistry Balancing Redox Reactions Assign ons and determine which elements change. Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the. Electrochemistry Balancing Redox Reactions.
From www.youtube.com
Balancing Redox Reactions in Acidic and Basic Conditions YouTube Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Assign ons and determine which elements change. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then. Electrochemistry Balancing Redox Reactions.
From www.youtube.com
Electrochemistry Made Easy Balancing Redox Reactions by the Half Electrochemistry Balancing Redox Reactions Assign ons and determine which elements change. Understand redox reactions in detail. Divide the reaction into half reactions using the elements which change as a guide. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion. Electrochemistry Balancing Redox Reactions.
From www.studypool.com
SOLUTION Chemistry Electrochemistry (Balancing Redox Reactions Electrochemistry Balancing Redox Reactions Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the. Electrochemistry Balancing Redox Reactions.
From worksheetlistee.z21.web.core.windows.net
Balance Redox Reaction Example Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Assign ons and determine which elements. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Balancing redox reactions PowerPoint Presentation, free download Electrochemistry Balancing Redox Reactions Understand redox reactions in detail. Assign ons and determine which elements change. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying. Electrochemistry Balancing Redox Reactions.
From pdfslide.net
(PPTX) 7.3 BALANCING REDOX REACTIONS USING OXIDATION NUMBERS UNIT 7 Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balancing a redox reaction. Electrochemistry Balancing Redox Reactions.
From slidetodoc.com
Electrochemistry Spontaneity of Redox Reactions 21 1 Electrochemistry Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Assign ons and determine which elements change. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions,. Electrochemistry Balancing Redox Reactions.
From www.slideshare.net
Tang 02 balancing redox reactions 2 Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Assign ons and determine which elements change. Balancing a redox reaction requires identifying the oxidation numbers in the. Electrochemistry Balancing Redox Reactions.
From www.youtube.com
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Divide the reaction into half reactions using the elements which change as a guide. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Assign ons and determine. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID6595241 Electrochemistry Balancing Redox Reactions Divide the reaction into half reactions using the elements which change as a guide. Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Balancing a redox reaction. Electrochemistry Balancing Redox Reactions.
From www.slideserve.com
PPT Unit 5 Part 2 Redox Reactions and Electrochemistry PowerPoint Electrochemistry Balancing Redox Reactions Balance each half reaction separately, leaving out water, hydrogen ion and hydroxide ion until the end and then combine the equations so that. Balancing a redox reaction requires identifying the oxidation numbers in the net ionic equation, breaking the equation into half reactions, adding. Divide the reaction into half reactions using the elements which change as a guide. Understand redox. Electrochemistry Balancing Redox Reactions.