What Are The Chemicals In Soap . The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The oil comes from an animal or plant, while the alkali is a chemical called lye. Surfactants are a common ingredient in detergents and other cleaning products. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Each soap molecule has a long. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Soap, by definition, is fat or oil mixed with an alkali.
from www.alamy.com
Soap, by definition, is fat or oil mixed with an alkali. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The oil comes from an animal or plant, while the alkali is a chemical called lye. Surfactants are a common ingredient in detergents and other cleaning products. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Each soap molecule has a long.
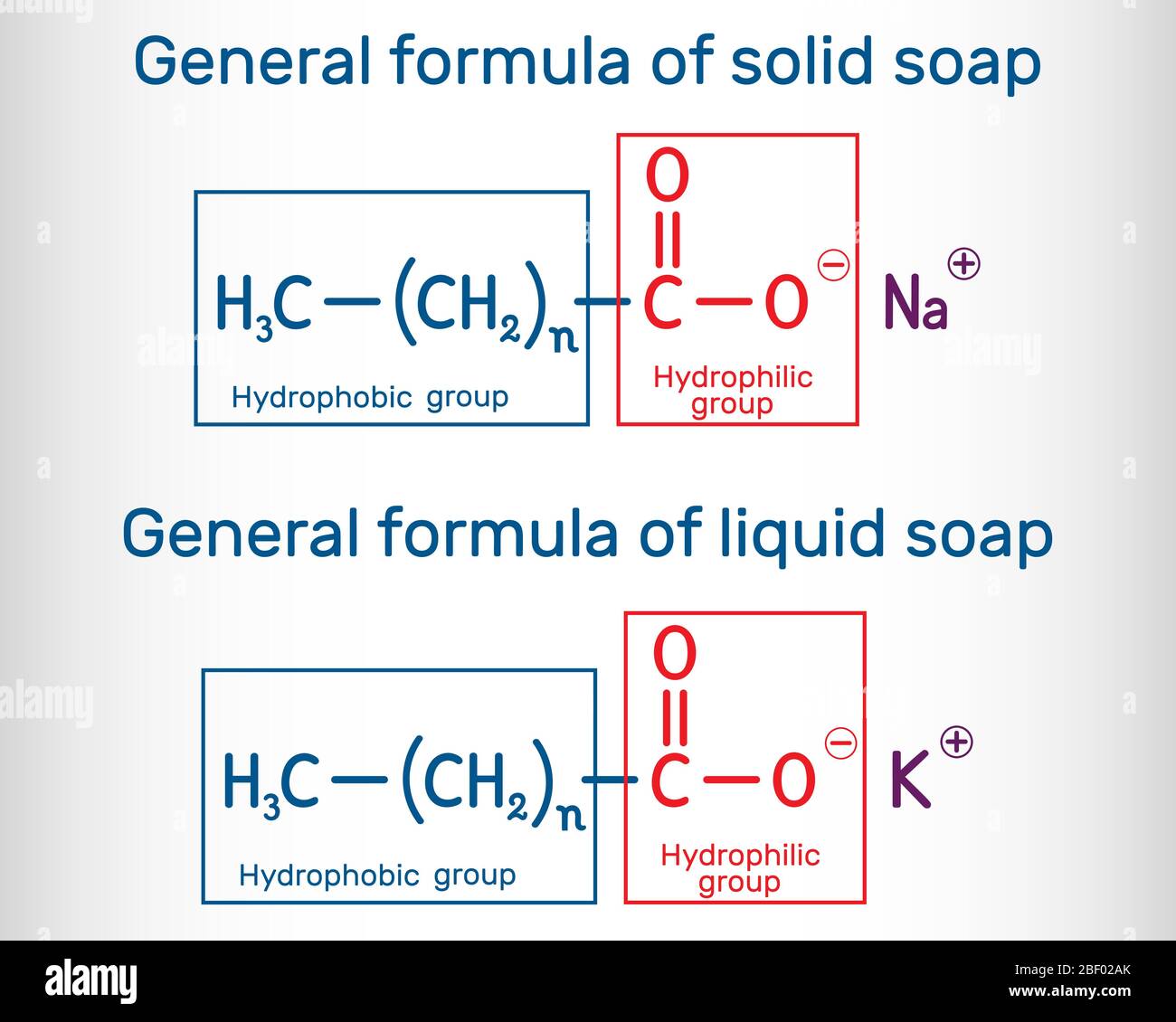
General formula of solid and liquid soap molecule. RCOONa, RCOOK
What Are The Chemicals In Soap The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Surfactants are a common ingredient in detergents and other cleaning products. The oil comes from an animal or plant, while the alkali is a chemical called lye. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soap, by definition, is fat or oil mixed with an alkali. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Each soap molecule has a long.
From www.webstaurantstore.com
Advantage Chemicals Bulk 5Gallon Liquid Dish Soap What Are The Chemicals In Soap Each soap molecule has a long. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Surfactants are a. What Are The Chemicals In Soap.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 What Are The Chemicals In Soap Soap, by definition, is fat or oil mixed with an alkali. The oil comes from an animal or plant, while the alkali is a chemical called lye. Surfactants are a common ingredient in detergents and other cleaning products. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap. What Are The Chemicals In Soap.
From www.medicalnewstoday.com
Harmful chemicals in soap Types, effects, and alternatives What Are The Chemicals In Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Surfactants are a common ingredient in detergents and other cleaning products. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap, by definition, is fat or oil mixed with an. What Are The Chemicals In Soap.
From blog.thesage.com
Soap & Detergent What is the difference? — Adventures With The Sage What Are The Chemicals In Soap Each soap molecule has a long. Soap, by definition, is fat or oil mixed with an alkali. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soaps are sodium or potassium fatty acids salts, produced from the. What Are The Chemicals In Soap.
From www.webstaurantstore.com
Advantage Chemicals 5 gallon / 640 oz. Liquid Dish Soap What Are The Chemicals In Soap Surfactants are a common ingredient in detergents and other cleaning products. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap, by definition, is fat or oil mixed with an alkali. Learn about the. What Are The Chemicals In Soap.
From www.pinterest.pt
Toxic chemicals have no place in soap! Make the switch to pure, artisan What Are The Chemicals In Soap Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Learn about the chemistry of cleaning and. What Are The Chemicals In Soap.
From www.slideshare.net
Chemistry of soaps What Are The Chemicals In Soap The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The oil comes from an animal or plant, while the alkali is a chemical called lye. Surfactants are a common ingredient in detergents and other cleaning products. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called. What Are The Chemicals In Soap.
From www.webstaurantstore.com
Advantage Chemicals 5 gallon / 640 oz. Concentrated Pot & Pan Soap What Are The Chemicals In Soap Soap, by definition, is fat or oil mixed with an alkali. The oil comes from an animal or plant, while the alkali is a chemical called lye. Each soap molecule has a long. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Learn about the chemistry of cleaning and how surfactants react with soil and. What Are The Chemicals In Soap.
From www.alamy.com
General formula of solid and liquid soap molecule. RCOONa, RCOOK What Are The Chemicals In Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Each soap molecule has a long. The oil comes from an animal or plant, while the alkali is a chemical called lye. Soap molecules have. What Are The Chemicals In Soap.
From www.scribd.com
Soap Soap Chemical Substances What Are The Chemicals In Soap Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soap, by definition, is fat or oil mixed with an alkali. Learn. What Are The Chemicals In Soap.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences What Are The Chemicals In Soap The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis. What Are The Chemicals In Soap.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab What Are The Chemicals In Soap Surfactants are a common ingredient in detergents and other cleaning products. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long. The chemistry of soap and detergents unveils the. What Are The Chemicals In Soap.
From dir.indiamart.com
Soap Chemicals Manufacturers & Suppliers in India What Are The Chemicals In Soap The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap, by definition, is fat or oil mixed with an alkali. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,.. What Are The Chemicals In Soap.
From jaimielistens.com
The Truth About the Chemicals in Your Soap What Are The Chemicals In Soap The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The oil comes from an animal or plant, while the alkali is a chemical called lye. Soap molecules have on one end what’s known as a. What Are The Chemicals In Soap.
From www.waca.msf.org
Advantage Chemicals 1 Gallon Foaming Hand Soap — Pristine Supply What Are The Chemicals In Soap Surfactants are a common ingredient in detergents and other cleaning products. Each soap molecule has a long. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The other end of the molecule is a nonpolar chain. What Are The Chemicals In Soap.
From www.pinterest.com
Best 14 NonToxic Hand Soaps Because Health Soap, Foaming hand soap What Are The Chemicals In Soap Each soap molecule has a long. Surfactants are a common ingredient in detergents and other cleaning products. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Soap, by definition, is fat or oil mixed with an alkali.. What Are The Chemicals In Soap.
From www.pinterest.ph
Hand washing with soap vector illustration. Educational explanation What Are The Chemicals In Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap, by definition, is fat or oil mixed with an alkali. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The other end of the molecule is a nonpolar chain of fatty. What Are The Chemicals In Soap.
From cen.acs.org
Periodic graphics Soap versus body wash What Are The Chemicals In Soap Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Surfactants are a common ingredient in detergents and other cleaning products. Each soap molecule has a long. Soap, by definition, is fat or oil mixed with an alkali. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The. What Are The Chemicals In Soap.
From www.thoughtco.com
Saponification Definition and Reaction What Are The Chemicals In Soap Surfactants are a common ingredient in detergents and other cleaning products. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness.. What Are The Chemicals In Soap.
From noahchemicals.com
Chemicals Used to Make Soap and Detergent Noah Chemicals What Are The Chemicals In Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The oil comes from an animal or plant, while the alkali is a chemical called lye. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap, by definition, is fat. What Are The Chemicals In Soap.
From www.slideshare.net
Chemistry of soaps What Are The Chemicals In Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. The oil comes from an animal or plant, while the alkali. What Are The Chemicals In Soap.
From abc13.com
FDA bans antiseptic chemicals from soaps ABC13 Houston What Are The Chemicals In Soap Each soap molecule has a long. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap, by definition, is fat or oil mixed with an alkali. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Learn about the chemistry of cleaning and how. What Are The Chemicals In Soap.
From www.webstaurantstore.com
Advantage Chemicals 32 oz. Concentrated Liquid Dish Soap What Are The Chemicals In Soap Soap, by definition, is fat or oil mixed with an alkali. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Learn about the chemistry of cleaning and how surfactants react. What Are The Chemicals In Soap.
From www.youtube.com
Chemistry 101 How does soap work? YouTube What Are The Chemicals In Soap Surfactants are a common ingredient in detergents and other cleaning products. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Each soap molecule has a long. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soaps are sodium or potassium fatty acids. What Are The Chemicals In Soap.
From www.reddit.com
This dish soap lists the purpose for each ingredient coolguides What Are The Chemicals In Soap Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Each soap molecule has a long. Surfactants are a common ingredient in detergents and other cleaning products. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soaps are sodium or potassium fatty acids salts, produced. What Are The Chemicals In Soap.
From www.webstaurantstore.com
Advantage Chemicals 32 oz. Liquid Dish Soap What Are The Chemicals In Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The oil comes from an animal or plant, while the alkali is a chemical called lye. Surfactants are a common ingredient in detergents and other cleaning products. Each soap molecule has a long. Learn about the chemistry of cleaning and. What Are The Chemicals In Soap.
From camachem.com
What Are the Best SoapMaking Chemicals? What Are The Chemicals In Soap Each soap molecule has a long. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. The oil comes from an animal or plant, while the alkali is a chemical called lye. Surfactants are a common ingredient in detergents and other cleaning products. Soap molecules have on one end what’s known as a polar salt,. What Are The Chemicals In Soap.
From thesoapmoleculeco.com
The Soap Molecule Co. What Are The Chemicals In Soap The oil comes from an animal or plant, while the alkali is a chemical called lye. Surfactants are a common ingredient in detergents and other cleaning products. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness.. What Are The Chemicals In Soap.
From www.thoughtco.com
How Saponification Makes Soap What Are The Chemicals In Soap Soap, by definition, is fat or oil mixed with an alkali. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The oil comes from an animal or plant, while the. What Are The Chemicals In Soap.
From www.webstaurantstore.com
Advantage Chemicals Bulk 5Gallon Liquid Dish Soap What Are The Chemicals In Soap The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Each soap molecule has a long. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Surfactants are a common ingredient. What Are The Chemicals In Soap.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents What Are The Chemicals In Soap Each soap molecule has a long. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. The oil. What Are The Chemicals In Soap.
From trueandnatural.com
Chemicals In Soap True and Natural Soap What Are The Chemicals In Soap The other end of the molecule is a nonpolar chain of fatty acids or hydrocarbons,. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long. Soap, by definition, is fat or oil mixed with an alkali. The oil comes from an animal or plant,. What Are The Chemicals In Soap.
From www.webstaurantstore.com
Advantage Chemicals 1 Gallon ReadytoUse Foaming Hand Soap What Are The Chemicals In Soap Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Surfactants are a common ingredient in detergents. What Are The Chemicals In Soap.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis What Are The Chemicals In Soap The chemistry of soap and detergents unveils the science that underlies our everyday cleanliness. Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Surfactants are a common ingredient in detergents and other cleaning products. The oil comes from an animal or plant, while the alkali is a chemical called lye. Each soap. What Are The Chemicals In Soap.
From cosmosmagazine.com
The chemistry of soap What Are The Chemicals In Soap Learn about the chemistry of cleaning and how surfactants react with soil and water to clean everything. Surfactants are a common ingredient in detergents and other cleaning products. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The other end of the molecule is a nonpolar chain of fatty acids. What Are The Chemicals In Soap.