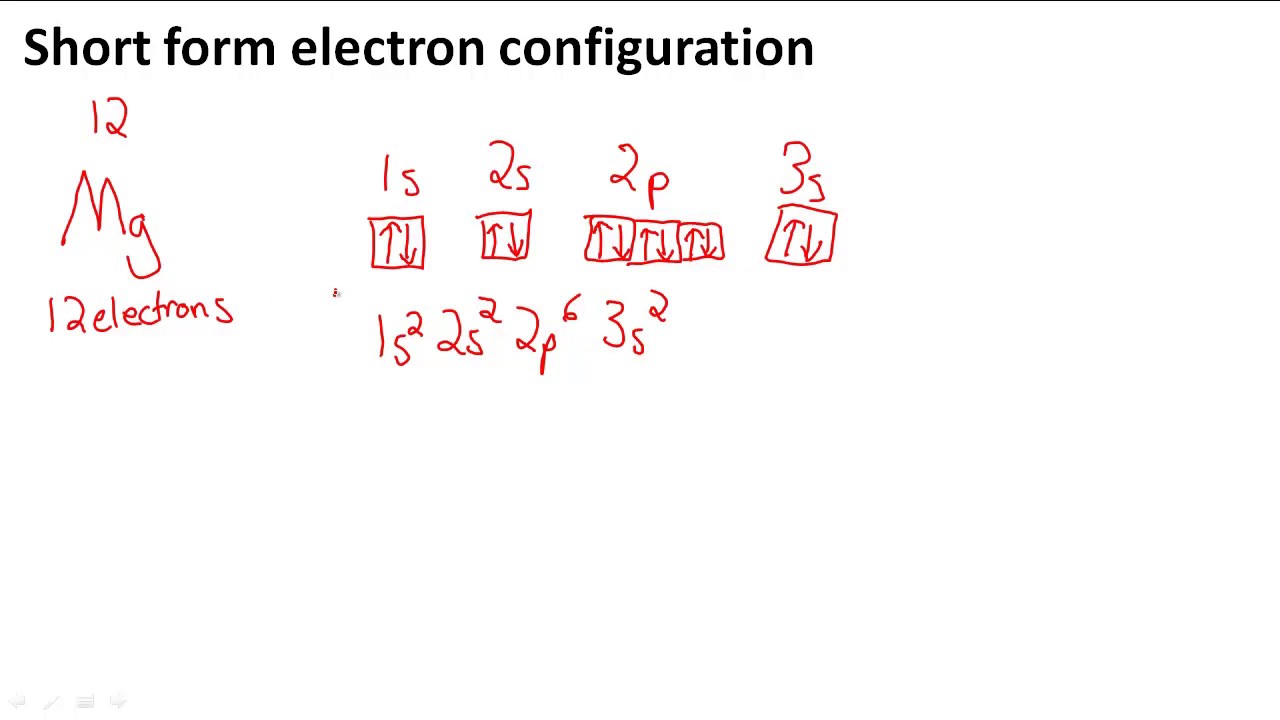
Magnesium Electron Orbitals . View rotating bohr models for all 118 elements. Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Get a free hd image of the. The ground state electron configuration of magnesium is important in understanding its. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals). When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. 119 rows access detailed info on all elements: Magnesium has 12 electrons arranged in three energy levels. Atomic mass, electron configurations, charges, and more. Magnesium is an alkaline earth metal with atomic number 12.
from utedzz.blogspot.com
Magnesium has two valence electrons in the 3s orbital. Magnesium has 12 electrons arranged in three energy levels. View rotating bohr models for all 118 elements. 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Magnesium is an alkaline earth metal with atomic number 12. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. The ground state electron configuration of magnesium is important in understanding its. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².
Periodic Table Magnesium Electron Configuration Periodic Table Timeline
Magnesium Electron Orbitals View rotating bohr models for all 118 elements. Magnesium has 12 electrons arranged in three energy levels. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals). Magnesium is an alkaline earth metal with atomic number 12. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Get a free hd image of the. 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118 elements. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. The ground state electron configuration of magnesium is important in understanding its. Magnesium has two valence electrons in the 3s orbital. Its electron configuration can be represented as 1s2 2s2 2p6 3s2.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Orbitals To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals). Get a free hd image. Magnesium Electron Orbitals.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Magnesium Electron Orbitals To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Magnesium has 12 electrons arranged in three energy levels. Atomic mass, electron configurations, charges, and more. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons. Magnesium Electron Orbitals.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence orbitals of an atom of Magnesium Electron Orbitals The ground state electron configuration of magnesium is important in understanding its. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Atomic mass, electron configurations, charges, and more. Get a free hd image of the. When we write the configuration we'll put. Magnesium Electron Orbitals.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium YouTube Magnesium Electron Orbitals Magnesium has 12 electrons arranged in three energy levels. Magnesium is an alkaline earth metal with atomic number 12. View rotating bohr models for all 118 elements. Get a free hd image of the. Atomic mass, electron configurations, charges, and more. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. When we. Magnesium Electron Orbitals.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Electron Orbitals Its electron configuration can be represented as 1s2 2s2 2p6 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital. Get a free hd image of the. 119 rows access detailed info on all elements: View rotating bohr models for all 118 elements. Electron configurations and orbital diagrams can be. Magnesium Electron Orbitals.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron configuration, chemical data, and Magnesium Electron Orbitals The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium is an alkaline earth metal with atomic number 12. View rotating bohr models for all 118 elements. Atomic mass, electron configurations, charges, and more. Magnesium has two valence electrons in the 3s orbital. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of. Magnesium Electron Orbitals.
From www.dreamstime.com
Atom Of Magnesium With Detailed Core And Its 12 Electrons Stock Illustration Illustration of Magnesium Electron Orbitals 119 rows access detailed info on all elements: Magnesium has two valence electrons in the 3s orbital. Atomic mass, electron configurations, charges, and more. Get a free hd image of the. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². View rotating bohr models for all 118 elements. When we write the configuration we'll put all 12 electrons in. Magnesium Electron Orbitals.
From mungfali.com
Magnesium Orbital Diagram Magnesium Electron Orbitals View rotating bohr models for all 118 elements. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Magnesium has 12 electrons arranged in three energy levels. Get a free hd image of the. 119 rows access detailed info on all elements: The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the. Magnesium Electron Orbitals.
From schematicfixunshared.z22.web.core.windows.net
Magnesium Orbital Diagram Magnesium Electron Orbitals Its electron configuration can be represented as 1s2 2s2 2p6 3s2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the. Magnesium Electron Orbitals.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Magnesium Electron Orbitals To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Magnesium has two valence electrons in the 3s orbital. 119 rows access detailed info on all elements: Magnesium is an alkaline earth metal with atomic number 12. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². When we write the configuration we'll. Magnesium Electron Orbitals.
From ar.inspiredpencil.com
Magnesium Atom Structure Magnesium Electron Orbitals View rotating bohr models for all 118 elements. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. 119 rows access detailed info on all elements: Magnesium has two valence electrons in the 3s orbital. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. The electron configuration of magnesium is 1s². Magnesium Electron Orbitals.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Electron Orbitals Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Magnesium has 12 electrons arranged in three energy levels. Magnesium has two valence electrons in the 3s orbital. Atomic mass, electron configurations, charges, and more. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Get a free hd image of the. 119 rows access detailed info on all. Magnesium Electron Orbitals.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Electron Orbitals Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Magnesium has two valence electrons in the 3s orbital. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same. Magnesium Electron Orbitals.
From guidedehartsculptures.z21.web.core.windows.net
Magnesium Electric Dot Diagram Magnesium Electron Orbitals Magnesium has 12 electrons arranged in three energy levels. The ground state electron configuration of magnesium is important in understanding its. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Atomic mass, electron configurations, charges, and more. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Electron configurations and orbital. Magnesium Electron Orbitals.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Periodic Table of the Elements Magnesium Electron Orbitals When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Magnesium has two valence electrons in the 3s orbital. Magnesium is an alkaline earth metal with atomic number 12. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. 119 rows access detailed info. Magnesium Electron Orbitals.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Electron Orbitals Magnesium has two valence electrons in the 3s orbital. View rotating bohr models for all 118 elements. Atomic mass, electron configurations, charges, and more. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 119 rows access detailed info on all. Magnesium Electron Orbitals.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Orbitals Atomic mass, electron configurations, charges, and more. Magnesium has 12 electrons arranged in three energy levels. View rotating bohr models for all 118 elements. Get a free hd image of the. The ground state electron configuration of magnesium is important in understanding its. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the. Magnesium Electron Orbitals.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Electron Orbitals To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Magnesium is an alkaline earth metal with atomic number 12. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. Atomic mass, electron configurations, charges, and more. Electron configurations and orbital diagrams can be. Magnesium Electron Orbitals.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Electron Orbitals Magnesium is an alkaline earth metal with atomic number 12. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals). Magnesium has 12 electrons arranged in three energy levels. Magnesium has two. Magnesium Electron Orbitals.
From guidemanualeruptivity.z14.web.core.windows.net
Number Of Orbitals In Magnesium Magnesium Electron Orbitals View rotating bohr models for all 118 elements. 119 rows access detailed info on all elements: Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals). When we write the configuration we'll. Magnesium Electron Orbitals.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Orbitals Get a free hd image of the. View rotating bohr models for all 118 elements. The ground state electron configuration of magnesium is important in understanding its. Magnesium has two valence electrons in the 3s orbital. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four. Magnesium Electron Orbitals.
From uzabytaodi.blogspot.com
41 orbital diagram of magnesium Wiring Diagrams Manual Magnesium Electron Orbitals Magnesium has two valence electrons in the 3s orbital. Magnesium is an alkaline earth metal with atomic number 12. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. View rotating bohr models for all 118 elements. Magnesium has 12 electrons. Magnesium Electron Orbitals.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Orbitals The ground state electron configuration of magnesium is important in understanding its. 119 rows access detailed info on all elements: When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same. Magnesium Electron Orbitals.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Orbitals Magnesium has 12 electrons arranged in three energy levels. Magnesium is an alkaline earth metal with atomic number 12. 119 rows access detailed info on all elements: When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Get a free hd image of the. Electron configurations and orbital diagrams can be. Magnesium Electron Orbitals.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Electron Orbitals Magnesium is an alkaline earth metal with atomic number 12. The ground state electron configuration of magnesium is important in understanding its. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 119 rows access detailed info on all elements: Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Get a free hd image of the. Magnesium has. Magnesium Electron Orbitals.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Orbitals 119 rows access detailed info on all elements: Magnesium is an alkaline earth metal with atomic number 12. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Atomic mass, electron configurations, charges, and more. Magnesium has 12 electrons arranged in three energy levels. Get a free hd image of the. To illustrate the magnesium orbital diagram, start by determining. Magnesium Electron Orbitals.
From quicycle.com
12. Magnesium The Quantum Bicycle Society Magnesium Electron Orbitals View rotating bohr models for all 118 elements. 119 rows access detailed info on all elements: The ground state electron configuration of magnesium is important in understanding its. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Magnesium has two valence electrons in the 3s orbital. Electron configurations and orbital diagrams can. Magnesium Electron Orbitals.
From enginedatanichered.z21.web.core.windows.net
Magnesium Atom Diagram Magnesium Electron Orbitals The ground state electron configuration of magnesium is important in understanding its. Magnesium has two valence electrons in the 3s orbital. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. View rotating bohr models for all 118 elements. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 119 rows access detailed info on all elements: To illustrate. Magnesium Electron Orbitals.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Electron Magnesium Electron Orbitals When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Get a free hd image of the. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. View rotating bohr models for all. Magnesium Electron Orbitals.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Electron Orbitals Magnesium has 12 electrons arranged in three energy levels. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. View rotating bohr models for all 118 elements. 119 rows access detailed info on all elements: Magnesium is an alkaline earth metal with atomic number 12. Atomic mass, electron configurations, charges, and. Magnesium Electron Orbitals.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Electron Orbitals The ground state electron configuration of magnesium is important in understanding its. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118 elements. Magnesium has two valence electrons in the 3s orbital. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. 119 rows access detailed info on all elements: When we write the configuration. Magnesium Electron Orbitals.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Protons are represented Magnesium Electron Orbitals Atomic mass, electron configurations, charges, and more. The ground state electron configuration of magnesium is important in understanding its. Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and hund’s rule (whenever. Magnesium Electron Orbitals.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Electron Orbitals Atomic mass, electron configurations, charges, and more. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. The ground state electron configuration of magnesium is important in understanding its. Magnesium has 12 electrons arranged in three energy levels. Magnesium is an alkaline earth metal with atomic number 12. The electron configuration of magnesium. Magnesium Electron Orbitals.
From www.breakingatom.com
Orbitals Periodic Table Magnesium Electron Orbitals View rotating bohr models for all 118 elements. When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the magnesium atom. To illustrate the magnesium orbital diagram, start by determining the number of electrons from the periodic table. Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two. Magnesium Electron Orbitals.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Electron Orbitals Electron configurations and orbital diagrams can be determined by applying the pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals). Its electron configuration can be represented as 1s2 2s2 2p6 3s2. Magnesium has 12 electrons arranged in three energy levels. Magnesium has. Magnesium Electron Orbitals.