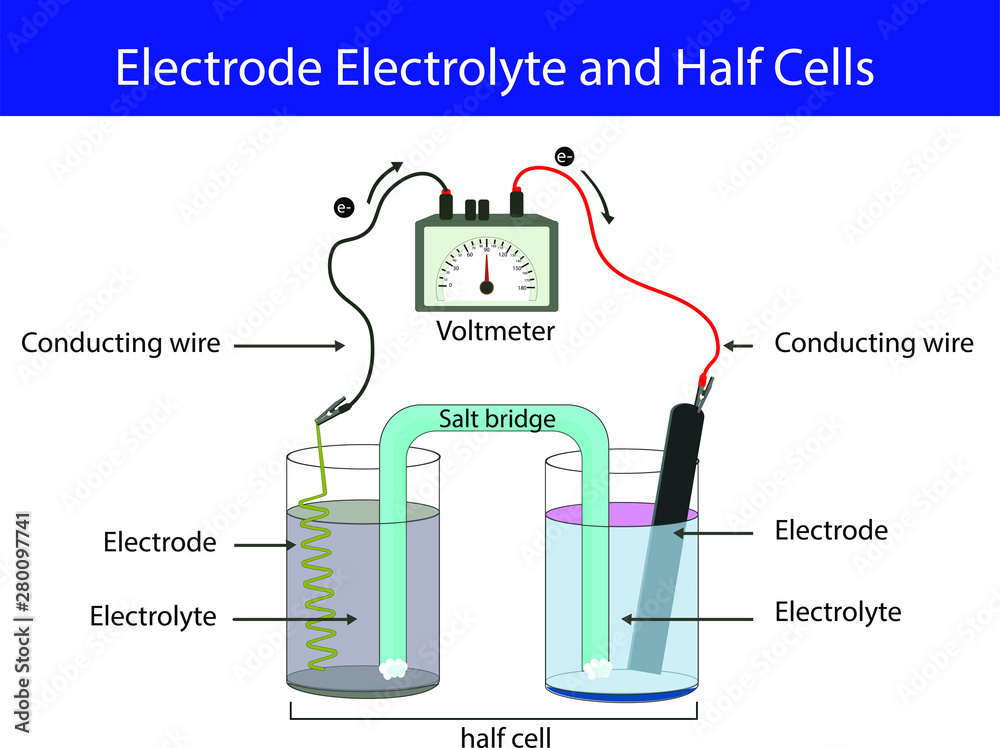
What Is Electrode Chemistry . Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. When the current leaves the electrodes it is known as the cathode and when the current. There are two types of electrodes, cathodes, and anodes. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are conductors by which electrons flow through to generate a current. It conducts current into and. An electrode by definition is a point where current enters and leaves the electrolyte.
from ar.inspiredpencil.com
Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. When the current leaves the electrodes it is known as the cathode and when the current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; There are two types of electrodes, cathodes, and anodes. An electrode by definition is a point where current enters and leaves the electrolyte. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. It conducts current into and. Electrodes are conductors by which electrons flow through to generate a current.
Electrode Chemistry
What Is Electrode Chemistry It conducts current into and. There are two types of electrodes, cathodes, and anodes. It conducts current into and. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode by definition is a point where current enters and leaves the electrolyte. When the current leaves the electrodes it is known as the cathode and when the current. Electrodes are conductors by which electrons flow through to generate a current. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Diagram What Is Electrode Chemistry There are two types of electrodes, cathodes, and anodes. It conducts current into and. Electrodes are conductors by which electrons flow through to generate a current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode is a conductor that is used to make contact with a nonmetallic part of a. What Is Electrode Chemistry.
From gradegorilla.com
GCSE Chemistry Cells Grade Gorilla What Is Electrode Chemistry An electrode by definition is a point where current enters and leaves the electrolyte. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. An electrode is a conductor that is used to make contact with a nonmetallic part. What Is Electrode Chemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is Electrode Chemistry When the current leaves the electrodes it is known as the cathode and when the current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. Electrodes are conductors by which electrons flow through to generate a current. An. What Is Electrode Chemistry.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) What Is Electrode Chemistry An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. When the current leaves the electrodes it is known as the cathode and when the current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are commonly used in electrochemical cells (see figure. What Is Electrode Chemistry.
From fphoto.photoshelter.com
science chemistry cathode ray tube Fundamental Photographs The Art of Science What Is Electrode Chemistry Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. There are two types of electrodes, cathodes, and anodes. Electrodes are conductors by which electrons flow through to generate a current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode is a conductor that is used. What Is Electrode Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrode Chemistry There are two types of electrodes, cathodes, and anodes. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. When the current leaves the electrodes it is known as the cathode and when the current. It conducts current into. What Is Electrode Chemistry.
From monomole.com
Standard hydrogen electrode Mono Mole What Is Electrode Chemistry An electrode by definition is a point where current enters and leaves the electrolyte. It conducts current into and. Electrodes are conductors by which electrons flow through to generate a current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode is a conductor that is used to make contact with. What Is Electrode Chemistry.
From www.youtube.com
Electrochemistry 5 Standard hydrogen electrode YouTube What Is Electrode Chemistry It conducts current into and. When the current leaves the electrodes it is known as the cathode and when the current. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. Electrode, electric conductor, usually metal, used as. What Is Electrode Chemistry.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts What Is Electrode Chemistry There are two types of electrodes, cathodes, and anodes. An electrode by definition is a point where current enters and leaves the electrolyte. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. It conducts current into and. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; When. What Is Electrode Chemistry.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry. YouTube What Is Electrode Chemistry Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. There are two types of electrodes, cathodes, and anodes. Electrodes are conductors by which electrons flow through to generate a current. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. It conducts current into and. Electrode, electric. What Is Electrode Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision What Is Electrode Chemistry Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. It conducts current into and. When the current leaves the electrodes it is known as the cathode and when the current. There are two types of electrodes, cathodes, and anodes. Electrodes are conductors by which electrons flow through to generate a current. An electrode is a. What Is Electrode Chemistry.
From chem.libretexts.org
6.2 Standard Electrode Potentials Chemistry LibreTexts What Is Electrode Chemistry An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. When the current leaves the electrodes it is known as the cathode and when the current. There are two types of electrodes, cathodes, and anodes. Electrodes are conductors by which electrons flow through to generate a current. Electrodes are commonly used in. What Is Electrode Chemistry.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors What Is Electrode Chemistry Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. When the current leaves the electrodes it is known as the cathode and when the current. There are two types of electrodes, cathodes, and anodes. It conducts current into and. An electrode by definition is a point where current enters and leaves the electrolyte. Electrode, electric. What Is Electrode Chemistry.
From www.snexplores.org
Explainer What is an electrode? What Is Electrode Chemistry An electrode by definition is a point where current enters and leaves the electrolyte. Electrodes are conductors by which electrons flow through to generate a current. There are two types of electrodes, cathodes, and anodes. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrode, electric conductor, usually metal, used as. What Is Electrode Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrode Chemistry Electrodes are conductors by which electrons flow through to generate a current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode by definition is a point where current enters and leaves the electrolyte. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. An electrode is. What Is Electrode Chemistry.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download Scientific Diagram What Is Electrode Chemistry Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are conductors by which electrons flow through to generate a current. When the current leaves the electrodes it is known as the cathode and. What Is Electrode Chemistry.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry What Is Electrode Chemistry An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. An electrode by definition is a point where current enters and leaves the electrolyte. There are two types of electrodes, cathodes, and anodes. Electrodes are conductors by which electrons flow through to generate a current. It conducts current into and. Electrode, electric. What Is Electrode Chemistry.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 4 electrode half equationss YouTube What Is Electrode Chemistry Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; It conducts current into and. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. When the current leaves the electrodes it is known as the cathode and when the current. There are two types of electrodes, cathodes, and. What Is Electrode Chemistry.
From stock.adobe.com
electrode potentials. standard hydrogen electrode. standard reduction electrode. chemistry What Is Electrode Chemistry There are two types of electrodes, cathodes, and anodes. Electrodes are conductors by which electrons flow through to generate a current. It conducts current into and. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting. What Is Electrode Chemistry.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts What Is Electrode Chemistry Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are conductors by which electrons flow through to generate a current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting. What Is Electrode Chemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is Electrode Chemistry Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are conductors by which electrons flow through to generate a current. There are two types of electrodes, cathodes, and anodes. An electrode is a conductor that is used. What Is Electrode Chemistry.
From www.youtube.com
Explain the origin of single electrode potential? Electrochemistry Physical Chemistry YouTube What Is Electrode Chemistry When the current leaves the electrodes it is known as the cathode and when the current. An electrode by definition is a point where current enters and leaves the electrolyte. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. It conducts current into and. Electrode, electric conductor, usually metal, used as. What Is Electrode Chemistry.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts What Is Electrode Chemistry There are two types of electrodes, cathodes, and anodes. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. It conducts current into and. When the current leaves the electrodes it is known as the cathode and when. What Is Electrode Chemistry.
From ar.inspiredpencil.com
Electrode Chemistry What Is Electrode Chemistry There are two types of electrodes, cathodes, and anodes. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are conductors by which electrons flow through to generate a current. An electrode by definition is a point where current enters and leaves the electrolyte. When the current leaves the electrodes it. What Is Electrode Chemistry.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12, Electro Chemistry What Is Electrode Chemistry It conducts current into and. When the current leaves the electrodes it is known as the cathode and when the current. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. Electrodes are conductors by which electrons flow through to generate a current. There are two types of electrodes, cathodes, and anodes. Electrode, electric conductor, usually. What Is Electrode Chemistry.
From www.slideserve.com
PPT Chemistry 142 Chapter 18 Electrochemistry PowerPoint Presentation ID1725688 What Is Electrode Chemistry Electrodes are conductors by which electrons flow through to generate a current. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode by definition is a point where current enters and leaves the electrolyte. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. It conducts current. What Is Electrode Chemistry.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts What Is Electrode Chemistry When the current leaves the electrodes it is known as the cathode and when the current. Electrodes are conductors by which electrons flow through to generate a current. There are two types of electrodes, cathodes, and anodes. An electrode by definition is a point where current enters and leaves the electrolyte. Electrodes are commonly used in electrochemical cells (see figure. What Is Electrode Chemistry.
From chem.libretexts.org
23.1 Reference Electrodes Chemistry LibreTexts What Is Electrode Chemistry An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. When the current leaves the electrodes it is known as the cathode and when the current. An electrode by definition is a point where current enters and leaves. What Is Electrode Chemistry.
From ar.inspiredpencil.com
Electrode Chemistry What Is Electrode Chemistry Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode by definition is a point where current enters and leaves the electrolyte. There are two types of electrodes, cathodes, and anodes. It conducts current into and. Electrodes. What Is Electrode Chemistry.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Chemistry Notes What Is Electrode Chemistry An electrode by definition is a point where current enters and leaves the electrolyte. When the current leaves the electrodes it is known as the cathode and when the current. There are two types of electrodes, cathodes, and anodes. Electrodes are conductors by which electrons flow through to generate a current. An electrode is a conductor that is used to. What Is Electrode Chemistry.
From byjus.com
What is an active electrode? Explain with the help of an example What Is Electrode Chemistry It conducts current into and. When the current leaves the electrodes it is known as the cathode and when the current. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are conductors by. What Is Electrode Chemistry.
From mungfali.com
Types Of Electrodes What Is Electrode Chemistry There are two types of electrodes, cathodes, and anodes. An electrode by definition is a point where current enters and leaves the electrolyte. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. When the current leaves the electrodes it is known as the cathode and when the current. Electrode, electric conductor,. What Is Electrode Chemistry.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts What Is Electrode Chemistry When the current leaves the electrodes it is known as the cathode and when the current. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are conductors by which electrons flow through to generate a current.. What Is Electrode Chemistry.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Chemistry Review What Is Electrode Chemistry It conducts current into and. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; When the current leaves the electrodes it is known as the cathode and when the current. An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. There are two types. What Is Electrode Chemistry.
From app.pandai.org
Standard Electrode Potential What Is Electrode Chemistry It conducts current into and. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode by definition is a point where current enters and leaves the electrolyte. Electrodes are commonly used in electrochemical cells (see figure 1), semiconductors like diodes, and. There are two types of electrodes, cathodes, and anodes. Electrodes. What Is Electrode Chemistry.