Dilution Factor Of 1/10 . The dilution factor is 1:10 in s:t notation. It’s also worth noting that. Dilution factor = v f / v i in this formula, v f represents the final volume of the. The dilution factor can be calculated using the following formula: Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. For example, if you have 100 ml of the. The dilution factor is calculated by dividing the initial. Dilution factor is the factor by which the stock solution is diluted. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. It may be expressed as the ratio of the volume of the final. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts. The dilution factor is often used as the denominator of a fraction. You have diluted the sample by a factor of 100. Dilution factor is the factor by which the stock solution is diluted. See below for the dilution factor equation.
from www.chegg.com
Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. The dilution factor is 1:10 in s:t notation. The dilution factor is calculated by dividing the initial. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts. Dilution factor is the factor by which the stock solution is diluted. Dilution factor = v f / v i in this formula, v f represents the final volume of the. See below for the dilution factor equation. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. It’s also worth noting that. It may be expressed as the ratio of the volume of the final.
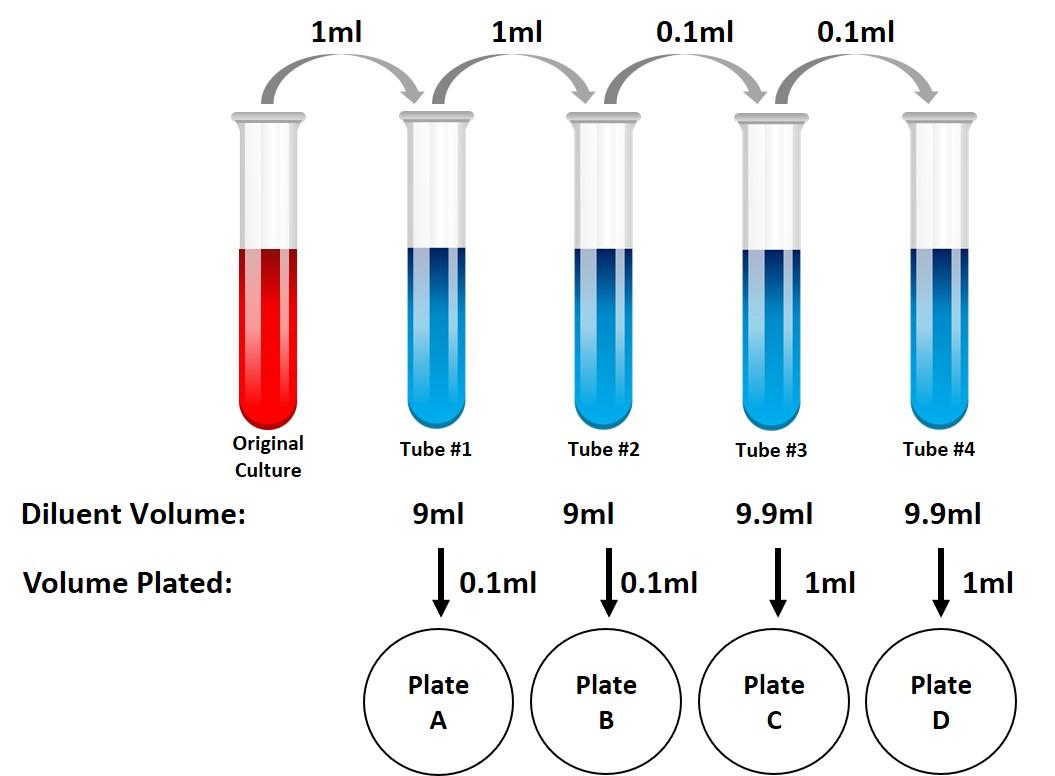
Solved You perform a serial dilution as shown in the figure
Dilution Factor Of 1/10 Dilution factor is the factor by which the stock solution is diluted. You have diluted the sample by a factor of 100. Dilution factor is the factor by which the stock solution is diluted. The dilution factor can be calculated using the following formula: See below for the dilution factor equation. Dilution factor = v f / v i in this formula, v f represents the final volume of the. The dilution factor is 1:10 in s:t notation. Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. Dilution factor is the factor by which the stock solution is diluted. It may be expressed as the ratio of the volume of the final. It quantifies the reduction in concentration resulting from dilution. The dilution factor is often used as the denominator of a fraction. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. For example, if you have 100 ml of the. It’s also worth noting that. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Factor Of 1/10 Dilution factor is the factor by which the stock solution is diluted. It’s also worth noting that. It quantifies the reduction in concentration resulting from dilution. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is 1:10 in s:t notation. The dilution factor is often used as the denominator of a fraction. You have. Dilution Factor Of 1/10.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Factor Of 1/10 Dilution factor is the factor by which the stock solution is diluted. It quantifies the reduction in concentration resulting from dilution. Dilution factor is the factor by which the stock solution is diluted. For example, if you have 100 ml of the. The dilution factor is often used as the denominator of a fraction. It may be expressed as the. Dilution Factor Of 1/10.
From www.freepik.com
Premium Vector Dilution factor formula science vector illustration Dilution Factor Of 1/10 It quantifies the reduction in concentration resulting from dilution. For example, if you have 100 ml of the. The dilution factor is calculated by dividing the initial. Dilution factor is the factor by which the stock solution is diluted. It may be expressed as the ratio of the volume of the final. Thus we have 10 cm 3 of stock. Dilution Factor Of 1/10.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Of 1/10 For example, if you have 100 ml of the. The dilution factor is calculated by dividing the initial. The dilution factor can be calculated using the following formula: Dilution factor is the factor by which the stock solution is diluted. It’s also worth noting that. The dilution factor is often used as the denominator of a fraction. Combine 9 parts. Dilution Factor Of 1/10.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Factor Of 1/10 Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. The dilution factor is calculated by dividing the initial. It quantifies the reduction in concentration resulting from dilution. For example, if you have 100 ml of the. It may be expressed as the ratio of the volume of the final. The dilution. Dilution Factor Of 1/10.
From quizdbbarnstorms.z21.web.core.windows.net
Cfu Stands For In Microbiology Dilution Factor Of 1/10 See below for the dilution factor equation. It quantifies the reduction in concentration resulting from dilution. The dilution factor is 1:10 in s:t notation. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. You have diluted the sample by a factor of 100. Dilution factor is the factor by which the stock solution is. Dilution Factor Of 1/10.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Factor Of 1/10 The dilution factor is often used as the denominator of a fraction. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts. Dilution factor is the factor by which the stock solution is diluted. See below for the dilution factor equation. It’s also worth noting that. Dilution factor is the factor by. Dilution Factor Of 1/10.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Factor Of 1/10 It quantifies the reduction in concentration resulting from dilution. The dilution factor can be calculated using the following formula: The dilution factor is 1:10 in s:t notation. Dilution factor = v f / v i in this formula, v f represents the final volume of the. Dilution factor is the factor by which the stock solution is diluted. It may. Dilution Factor Of 1/10.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Factor Of 1/10 It quantifies the reduction in concentration resulting from dilution. The dilution factor is 1:10 in s:t notation. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts. It may be expressed as the ratio of the volume of the final. For example, if you have 100 ml of the. Dilution factor =. Dilution Factor Of 1/10.
From quincyafebanks.blogspot.com
How to Calculate Dilution Factor QuincyafeBanks Dilution Factor Of 1/10 The dilution factor can be calculated using the following formula: Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. The dilution factor is calculated by dividing the initial. For example, if you have 100 ml of the. Dilution factor = v f / v i in this formula, v f represents. Dilution Factor Of 1/10.
From www.chegg.com
Solved You perform a serial dilution as shown in the figure Dilution Factor Of 1/10 You have diluted the sample by a factor of 100. The dilution factor is often used as the denominator of a fraction. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. The dilution factor is calculated by dividing the initial. Dilution factor is the factor by which the stock solution is diluted. The dilution. Dilution Factor Of 1/10.
From bdaschools.weebly.com
bdaschools Blog Dilution Factor Of 1/10 For example, if you have 100 ml of the. The dilution factor can be calculated using the following formula: The dilution factor is 1:10 in s:t notation. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. It’s also worth noting that. Thus we have 10 cm 3 of stock solution that now makes up. Dilution Factor Of 1/10.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Factor Of 1/10 Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. It may be expressed as the ratio of the volume of the final. Dilution factor is the factor by which the stock solution is diluted. It quantifies the reduction in concentration resulting from dilution. You have diluted the sample by a factor. Dilution Factor Of 1/10.
From support.axionbio.com
Understanding of Cell Sample Dilution Factor Dilution Factor Of 1/10 For example, if you have 100 ml of the. It quantifies the reduction in concentration resulting from dilution. Dilution factor is the factor by which the stock solution is diluted. The dilution factor can be calculated using the following formula: Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. The dilution. Dilution Factor Of 1/10.
From www.youtube.com
AS Biology How to calculate serial and simple dilutions YouTube Dilution Factor Of 1/10 The dilution factor is calculated by dividing the initial. The dilution factor is often used as the denominator of a fraction. It quantifies the reduction in concentration resulting from dilution. For example, if you have 100 ml of the. Dilution factor = v f / v i in this formula, v f represents the final volume of the. For example,. Dilution Factor Of 1/10.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution Factor Of 1/10 You have diluted the sample by a factor of 100. Dilution factor is the factor by which the stock solution is diluted. For example, if you have 100 ml of the. The dilution factor can be calculated using the following formula: For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. Dilution factor is the. Dilution Factor Of 1/10.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Factor Of 1/10 See below for the dilution factor equation. Dilution factor is the factor by which the stock solution is diluted. The dilution factor can be calculated using the following formula: Dilution factor = v f / v i in this formula, v f represents the final volume of the. You have diluted the sample by a factor of 100. For example,. Dilution Factor Of 1/10.
From studylib.net
Dilution Ratios Table Dilution Factor Of 1/10 The dilution factor is 1:10 in s:t notation. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. For example, if you have 100 ml of the. Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. The dilution factor can be calculated using the following formula:. Dilution Factor Of 1/10.
From bio.libretexts.org
1.8 Serial Dilutions and Standard Curve Biology LibreTexts Dilution Factor Of 1/10 You have diluted the sample by a factor of 100. The dilution factor is 1:10 in s:t notation. It quantifies the reduction in concentration resulting from dilution. The dilution factor is often used as the denominator of a fraction. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is calculated by dividing the initial.. Dilution Factor Of 1/10.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Factor Of 1/10 It quantifies the reduction in concentration resulting from dilution. The dilution factor can be calculated using the following formula: See below for the dilution factor equation. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts. The dilution factor is calculated by dividing the initial. For example, if you have 100 ml. Dilution Factor Of 1/10.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Factor Of 1/10 For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. It quantifies the reduction in concentration resulting from dilution. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is calculated by dividing the initial. Combine 9 parts of diluent with 1 part of the stock solution, making a total. Dilution Factor Of 1/10.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Factor Of 1/10 The dilution factor can be calculated using the following formula: Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. For example, if you have 100 ml of the. See below for the dilution factor equation. The dilution factor is often used as the denominator of a fraction. It quantifies the reduction. Dilution Factor Of 1/10.
From www.youtube.com
Calculating Dilution Factor YouTube Dilution Factor Of 1/10 The dilution factor is often used as the denominator of a fraction. Dilution factor is the factor by which the stock solution is diluted. It quantifies the reduction in concentration resulting from dilution. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. The dilution factor is calculated by dividing the initial. Dilution factor =. Dilution Factor Of 1/10.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Factor Of 1/10 Dilution factor is the factor by which the stock solution is diluted. Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. It may be expressed as the ratio of the volume of the final. The dilution factor is calculated by dividing the initial. See below for the dilution factor equation. It. Dilution Factor Of 1/10.
From bio.libretexts.org
15 Determination of Bacterial Numbers Biology LibreTexts Dilution Factor Of 1/10 It may be expressed as the ratio of the volume of the final. The dilution factor is 1:10 in s:t notation. For example, if you have 100 ml of the. Dilution factor is the factor by which the stock solution is diluted. Dilution factor = v f / v i in this formula, v f represents the final volume of. Dilution Factor Of 1/10.
From www.slideshare.net
Serial dilution Dilution Factor Of 1/10 It may be expressed as the ratio of the volume of the final. It quantifies the reduction in concentration resulting from dilution. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts. The dilution factor is often used as the denominator of a fraction. The dilution factor is 1:10 in s:t notation.. Dilution Factor Of 1/10.
From denisseqibowen.blogspot.com
How to Calculate Dilution Factor DenisseqiBowen Dilution Factor Of 1/10 The dilution factor is often used as the denominator of a fraction. Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. The dilution factor can be calculated using the following formula: Dilution factor = v f / v i in this formula, v f represents the final volume of the. It. Dilution Factor Of 1/10.
From www.transtutors.com
(Get Answer) Table 1 Serial Dilutions By A Factor Of 10 Volume (ML Dilution Factor Of 1/10 Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is often used as the denominator of a fraction. See below for. Dilution Factor Of 1/10.
From www.scribd.com
DilutionFactor Dilution Factor Of 1/10 It quantifies the reduction in concentration resulting from dilution. See below for the dilution factor equation. It may be expressed as the ratio of the volume of the final. Dilution factor is the factor by which the stock solution is diluted. You have diluted the sample by a factor of 100. Dilution factor is the factor by which the stock. Dilution Factor Of 1/10.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Factor Of 1/10 It quantifies the reduction in concentration resulting from dilution. The dilution factor can be calculated using the following formula: Dilution factor is the factor by which the stock solution is diluted. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. Dilution factor is the factor by which the stock solution is diluted. It may. Dilution Factor Of 1/10.
From www.biologyexams4u.com
How to Calculate CFU per ml of a Bacterial sample? In simple 3 steps Dilution Factor Of 1/10 You have diluted the sample by a factor of 100. The dilution factor can be calculated using the following formula: Dilution factor is the factor by which the stock solution is diluted. Combine 9 parts of diluent with 1 part of the stock solution, making a total of 10 parts. Dilution factor is the factor by which the stock solution. Dilution Factor Of 1/10.
From www.pdfprof.com
how to calculate dilution factor Dilution Factor Of 1/10 Dilution factor is the factor by which the stock solution is diluted. For example, if 100 ml of a stock solution is diluted with solvent/diluent to a. Thus we have 10 cm 3 of stock solution that now makes up a 100 cm 3 solution. You have diluted the sample by a factor of 100. The dilution factor can be. Dilution Factor Of 1/10.
From mungfali.com
10 Fold Serial Dilution Dilution Factor Of 1/10 The dilution factor is often used as the denominator of a fraction. The dilution factor can be calculated using the following formula: You have diluted the sample by a factor of 100. Dilution factor is the factor by which the stock solution is diluted. The dilution factor is 1:10 in s:t notation. It quantifies the reduction in concentration resulting from. Dilution Factor Of 1/10.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Factor Of 1/10 You have diluted the sample by a factor of 100. The dilution factor is often used as the denominator of a fraction. The dilution factor is calculated by dividing the initial. See below for the dilution factor equation. Dilution factor is the factor by which the stock solution is diluted. It’s also worth noting that. The dilution factor is 1:10. Dilution Factor Of 1/10.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Factor Of 1/10 Dilution factor is the factor by which the stock solution is diluted. You have diluted the sample by a factor of 100. The dilution factor can be calculated using the following formula: It’s also worth noting that. For example, if you have 100 ml of the. Thus we have 10 cm 3 of stock solution that now makes up a. Dilution Factor Of 1/10.