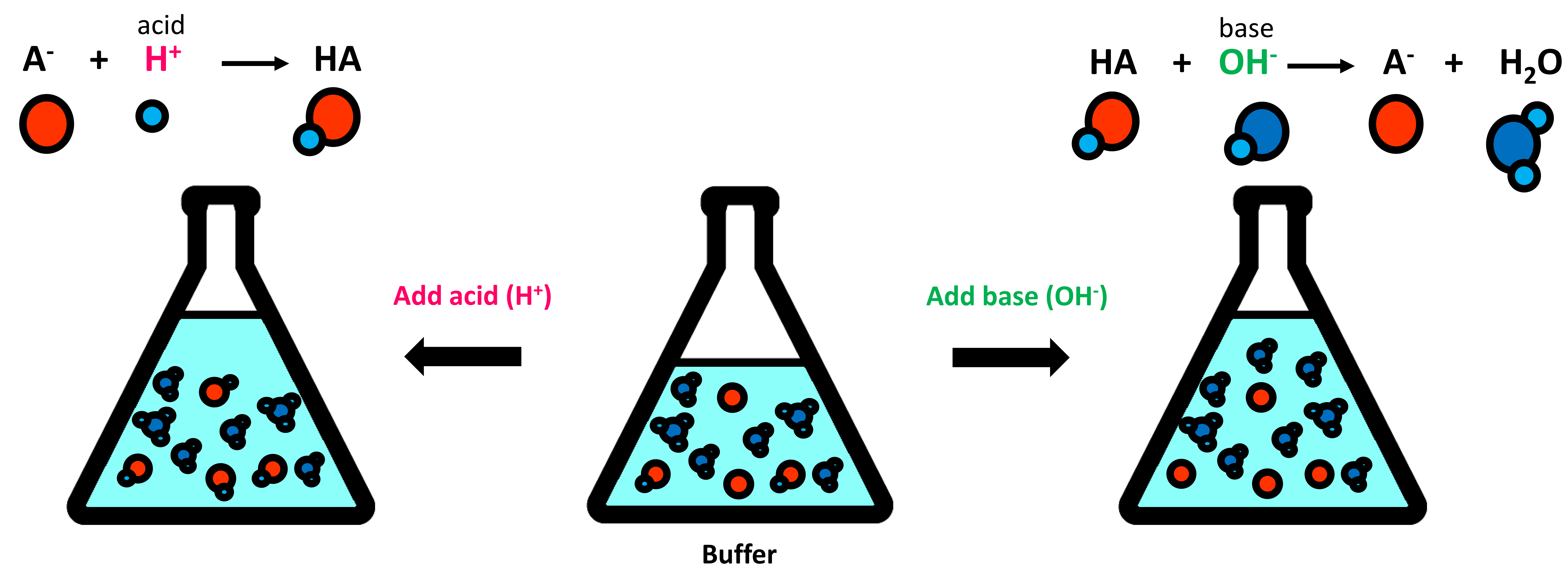
What Is A Natural Buffer . A solution whose ph is not altered to any great extent by the addition of small quantities of. A buffering agent is a weak acid or weak base that helps maintain the ph of an. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer (or buffered) solution is one that resists a. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. a buffer is an aqueous solution that has a highly stable ph. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. introduction to buffers. what is buffer in chemistry?
from deporecipe.co
the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer (or buffered) solution is one that resists a. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is an aqueous solution that has a highly stable ph. A buffering agent is a weak acid or weak base that helps maintain the ph of an. introduction to buffers. what is buffer in chemistry?
10x Protein Running Buffer Recipe Deporecipe.co
What Is A Natural Buffer buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. a buffer is an aqueous solution that has a highly stable ph. introduction to buffers. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. what is buffer in chemistry? A buffering agent is a weak acid or weak base that helps maintain the ph of an. A buffer (or buffered) solution is one that resists a. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution whose ph is not altered to any great extent by the addition of small quantities of. the mechanism involves a buffer, a solution that resists dramatic changes in ph.
From www.chegg.com
Solved One of the most important natural buffers includes What Is A Natural Buffer the mechanism involves a buffer, a solution that resists dramatic changes in ph. what is buffer in chemistry? A buffering agent is a weak acid or weak base that helps maintain the ph of an. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. a buffer is an aqueous solution. What Is A Natural Buffer.
From www.slideserve.com
PPT Chapter 13 AcidBase Balance PowerPoint Presentation, free What Is A Natural Buffer A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. the mechanism involves a buffer, a solution that resists dramatic changes in. What Is A Natural Buffer.
From wetlandinfo.des.qld.gov.au
Vegetated buffers and swales (Department of Environment and Science) What Is A Natural Buffer the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffering agent is a weak acid or weak base that helps maintain the ph of an. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. two. What Is A Natural Buffer.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is A Natural Buffer A buffer (or buffered) solution is one that resists a. the mechanism involves a buffer, a solution that resists dramatic changes in ph. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. what is buffer in chemistry? two natural buffers—carbonate and bicarbonate—play a. What Is A Natural Buffer.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Natural Buffer a buffer is an aqueous solution that has a highly stable ph. introduction to buffers. A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain. What Is A Natural Buffer.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is A Natural Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is an aqueous solution that has a highly stable ph. A buffering agent is a weak acid or weak base that helps maintain the ph of an. the mechanism involves a buffer, a. What Is A Natural Buffer.
From woodlandinfo.org
Riparian Buffers Agroforestry for Any Property UWMadison Extension What Is A Natural Buffer A buffering agent is a weak acid or weak base that helps maintain the ph of an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. a buffer is an aqueous solution that has a highly stable ph. A buffer is a solution that can resist ph change upon the. What Is A Natural Buffer.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your What Is A Natural Buffer introduction to buffers. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. A buffer (or buffered) solution is one that resists a. A buffering agent is a weak acid or weak base that helps maintain the ph of an. A solution whose ph is not altered to any great extent by the. What Is A Natural Buffer.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Is A Natural Buffer the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. introduction to buffers. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer (or buffered) solution is one that resists a. a buffer is a solution that maintains the. What Is A Natural Buffer.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID What Is A Natural Buffer a buffer is an aqueous solution that has a highly stable ph. A buffering agent is a weak acid or weak base that helps maintain the ph of an. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution whose ph is not altered to any great extent by the addition of small. What Is A Natural Buffer.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co What Is A Natural Buffer what is buffer in chemistry? the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer (or buffered) solution is one that resists a. the mechanism involves a buffer, a solution that resists dramatic changes in ph. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the. What Is A Natural Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Natural Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. A buffer (or buffered) solution is one that resists a. the mechanism involves a buffer, a solution that resists dramatic changes in ph. . What Is A Natural Buffer.
From www.youtube.com
An Example of Natural Buffer YouTube What Is A Natural Buffer A buffer (or buffered) solution is one that resists a. A buffering agent is a weak acid or weak base that helps maintain the ph of an. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph. What Is A Natural Buffer.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers What Is A Natural Buffer A buffer (or buffered) solution is one that resists a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. a buffer is a solution that maintains the stability of a system’s ph level. What Is A Natural Buffer.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID What Is A Natural Buffer A solution whose ph is not altered to any great extent by the addition of small quantities of. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is buffer in chemistry? A buffer (or buffered) solution is one that resists a. the mechanism involves a buffer, a. What Is A Natural Buffer.
From www.okbeli.com.my
Natural Buffer Solution 200ml OkBeli What Is A Natural Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. introduction to buffers. the mechanism involves a buffer, a solution that. What Is A Natural Buffer.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Natural Buffer introduction to buffers. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution whose ph is not altered to any great. What Is A Natural Buffer.
From watershedfriendlypa.org
Streamside Buffers WatershedFriendly PA What Is A Natural Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is an aqueous solution that has a highly stable ph. introduction to buffers. A. What Is A Natural Buffer.
From snohomishcd.org
Harvestable Riparian Buffers — Snohomish Conservation District What Is A Natural Buffer introduction to buffers. A solution whose ph is not altered to any great extent by the addition of small quantities of. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that. What Is A Natural Buffer.
From www.youtube.com
How Chemical Buffers Work & Buffers in Natural Systems // HSC Chemistry What Is A Natural Buffer A buffering agent is a weak acid or weak base that helps maintain the ph of an. what is buffer in chemistry? A buffer (or buffered) solution is one that resists a. introduction to buffers. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph. What Is A Natural Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID7055849 What Is A Natural Buffer what is buffer in chemistry? the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is an aqueous solution that has a highly stable ph. two natural buffers—carbonate and bicarbonate—play a vital. What Is A Natural Buffer.
From www.musimmas.com
How Do Riparian Buffers Protect the Environment? Musim Mas What Is A Natural Buffer the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. a buffer is an aqueous solution that has a highly stable ph. what is buffer in chemistry? A buffer (or buffered) solution is one that resists a. A solution whose ph is not altered to any great extent by the. What Is A Natural Buffer.
From www.slideshare.net
Acid base balance What Is A Natural Buffer what is buffer in chemistry? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffering agent is a weak acid or weak base that helps maintain the ph of an. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. What Is A Natural Buffer.
From natureworkspark.org
Riparian Buffer NatureWorksPark What Is A Natural Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. A buffer (or buffered) solution is one that resists a. the buffer systems. What Is A Natural Buffer.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses What Is A Natural Buffer A buffering agent is a weak acid or weak base that helps maintain the ph of an. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer (or buffered) solution is one that resists a. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate. What Is A Natural Buffer.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Natural Buffer buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. the mechanism involves a buffer, a solution that resists dramatic changes in ph. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. What Is A Natural Buffer.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Natural Buffer what is buffer in chemistry? two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is a. What Is A Natural Buffer.
From www.youtube.com
Buffer Systems Natural Buffer Systems YouTube What Is A Natural Buffer introduction to buffers. what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer, in chemistry, solution usually containing an acid. What Is A Natural Buffer.
From www.thoughtco.com
What Is a Buffer and How Does It Work? What Is A Natural Buffer the mechanism involves a buffer, a solution that resists dramatic changes in ph. introduction to buffers. A buffer (or buffered) solution is one that resists a. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. the buffer systems functioning in blood plasma include. What Is A Natural Buffer.
From www.slideshare.net
Buffer system What Is A Natural Buffer introduction to buffers. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the mechanism involves a buffer, a solution that resists dramatic changes in ph. what is buffer in chemistry? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to. What Is A Natural Buffer.
From www.youtube.com
8. Natural buffer systems (HSC chemistry) YouTube What Is A Natural Buffer a buffer is an aqueous solution that has a highly stable ph. A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. two natural buffers—carbonate and. What Is A Natural Buffer.
From www.newswise.com
What Are Riparian Buffer Strips? Newswise News for Journalists What Is A Natural Buffer the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. A buffer (or buffered) solution is one that resists a. two natural buffers—carbonate and bicarbonate—play a vital role in regulating the body's blood ph levels. A buffer is a solution that can resist ph change upon the addition of an acidic. What Is A Natural Buffer.
From bwsr.state.mn.us
Buffer and Alternative Practices Benefits MN Board of Water, Soil What Is A Natural Buffer the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered) solution is one that resists a. what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. A buffer is a solution that can resist ph change upon the. What Is A Natural Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Natural Buffer the mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffering agent is a weak acid or weak base that helps maintain the ph of an. what is buffer in chemistry? A buffer (or buffered) solution is one that resists a. introduction to buffers. A buffer is a solution that can resist ph. What Is A Natural Buffer.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Is A Natural Buffer A buffer (or buffered) solution is one that resists a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution whose ph is not altered to any great extent by the addition of small quantities of. a buffer is a solution that maintains the stability of a system’s. What Is A Natural Buffer.