Chlorine To Chloride Ions Half Equation . if we check the charge on both sides of the equation, we see they are the same—2+. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The slideshow shows what happens when. (in reality, this positive charge is. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. Chloride ions from a chlorine molecule:. half equations can be used to represent these reactions (higher tier): The reaction between chlorine and iron. In the process, the chlorine is reduced to chloride ions.
from www.numerade.com
Chloride ions from a chlorine molecule:. In the process, the chlorine is reduced to chloride ions. if we check the charge on both sides of the equation, we see they are the same—2+. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. The reaction between chlorine and iron. half equations can be used to represent these reactions (higher tier): cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The slideshow shows what happens when. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: (in reality, this positive charge is.
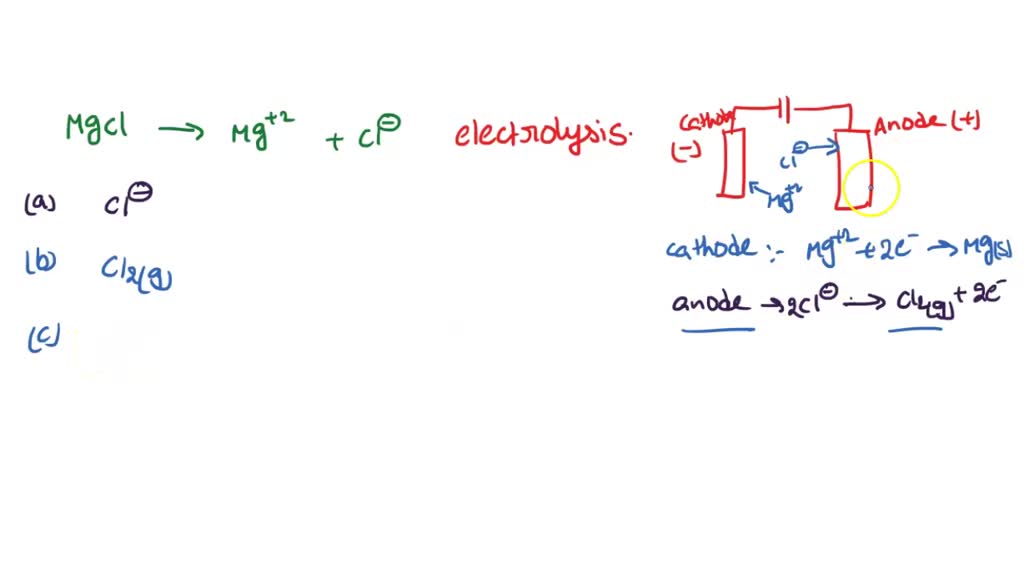
SOLVED Magnesium and chlorine can be made by the electrolysis of
Chlorine To Chloride Ions Half Equation each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: (in reality, this positive charge is. Chloride ions from a chlorine molecule:. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. The slideshow shows what happens when. The reaction between chlorine and iron. if we check the charge on both sides of the equation, we see they are the same—2+. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. half equations can be used to represent these reactions (higher tier): In the process, the chlorine is reduced to chloride ions.
From www.shalom-education.com
Reactions of Halogens GCSE Chemistry Revision Chlorine To Chloride Ions Half Equation if we check the charge on both sides of the equation, we see they are the same—2+. (in reality, this positive charge is. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. In the process, the chlorine is reduced to chloride ions. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you. Chlorine To Chloride Ions Half Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine To Chloride Ions Half Equation chlorine gas oxidizes iron(ii) ions to iron(iii) ions. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: The reaction between chlorine and iron. (in reality, this positive charge is. half equations can be used to represent these reactions (higher tier): Chloride ions from a chlorine. Chlorine To Chloride Ions Half Equation.
From www.youtube.com
Half Equations Tutorial YouTube Chlorine To Chloride Ions Half Equation half equations can be used to represent these reactions (higher tier): if we check the charge on both sides of the equation, we see they are the same—2+. The reaction between chlorine and iron. (in reality, this positive charge is. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns. Chlorine To Chloride Ions Half Equation.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Chlorine To Chloride Ions Half Equation each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: In the process, the chlorine is reduced to chloride ions. half equations can be used to represent these reactions (higher tier): cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction. Chlorine To Chloride Ions Half Equation.
From pediaa.com
Difference Between Chlorine and Chloride Definition, Properties Chlorine To Chloride Ions Half Equation each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. half equations can be used to represent these reactions (higher tier): chlorine gas. Chlorine To Chloride Ions Half Equation.
From shiken.ai
Chlorine Reactions Physical Chemistry Chlorine To Chloride Ions Half Equation chlorine gas oxidizes iron(ii) ions to iron(iii) ions. half equations can be used to represent these reactions (higher tier): Chloride ions from a chlorine molecule:. In the process, the chlorine is reduced to chloride ions. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: . Chlorine To Chloride Ions Half Equation.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine To Chloride Ions Half Equation The slideshow shows what happens when. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: (in reality, this positive charge is. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. chlorine gas. Chlorine To Chloride Ions Half Equation.
From www.essentialchemicalindustry.org
Chlorine Chlorine To Chloride Ions Half Equation Chloride ions from a chlorine molecule:. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. half equations can be used to represent these reactions (higher tier): each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write. Chlorine To Chloride Ions Half Equation.
From www.toppr.com
In passing chlorine gas through a concentrated solution of alkali, we Chlorine To Chloride Ions Half Equation if we check the charge on both sides of the equation, we see they are the same—2+. (in reality, this positive charge is. In the process, the chlorine is reduced to chloride ions. half equations can be used to represent these reactions (higher tier): each chlorine atom in a chlorine molecule gains an electron (it is being. Chlorine To Chloride Ions Half Equation.
From www.numerade.com
SOLVED In basic solution, molecular chlorine, Cl 2 , reacts with Chlorine To Chloride Ions Half Equation The reaction between chlorine and iron. Chloride ions from a chlorine molecule:. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: chlorine gas oxidizes iron(ii) ions to iron(iii) ions. In the process, the chlorine is reduced to chloride ions. (in reality, this positive charge is. The. Chlorine To Chloride Ions Half Equation.
From fyoboxomd.blob.core.windows.net
Chlorine Has 7 Valence Electrons. What Is The Charge Of A Chlorine Ion Chlorine To Chloride Ions Half Equation Chloride ions from a chlorine molecule:. (in reality, this positive charge is. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: In the process, the chlorine is reduced to chloride ions. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. half equations can be used to. Chlorine To Chloride Ions Half Equation.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Chlorine To Chloride Ions Half Equation if we check the charge on both sides of the equation, we see they are the same—2+. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: The reaction between chlorine and iron. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq). Chlorine To Chloride Ions Half Equation.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine To Chloride Ions Half Equation The reaction between chlorine and iron. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. (in reality, this positive charge is. Chloride ions from a chlorine molecule:. half equations can be used to represent these reactions. Chlorine To Chloride Ions Half Equation.
From www.numerade.com
SOLVED Magnesium and chlorine can be made by the electrolysis of Chlorine To Chloride Ions Half Equation The slideshow shows what happens when. half equations can be used to represent these reactions (higher tier): Chloride ions from a chlorine molecule:. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. The reaction between chlorine and iron. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half. Chlorine To Chloride Ions Half Equation.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine To Chloride Ions Half Equation half equations can be used to represent these reactions (higher tier): In the process, the chlorine is reduced to chloride ions. The slideshow shows what happens when. Chloride ions from a chlorine molecule:. (in reality, this positive charge is. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and. Chlorine To Chloride Ions Half Equation.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine To Chloride Ions Half Equation Chloride ions from a chlorine molecule:. half equations can be used to represent these reactions (higher tier): if we check the charge on both sides of the equation, we see they are the same—2+. The slideshow shows what happens when. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns. Chlorine To Chloride Ions Half Equation.
From en.ppt-online.org
Uses of chlorine and its compounds online presentation Chlorine To Chloride Ions Half Equation The slideshow shows what happens when. (in reality, this positive charge is. The reaction between chlorine and iron. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. Chloride ions from a chlorine molecule:. In the process, the. Chlorine To Chloride Ions Half Equation.
From www.tes.com
Oxidation States and Complex half Equations Lesson AS Teaching Resources Chlorine To Chloride Ions Half Equation The reaction between chlorine and iron. The slideshow shows what happens when. (in reality, this positive charge is. Chloride ions from a chlorine molecule:. half equations can be used to represent these reactions (higher tier): each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: In the. Chlorine To Chloride Ions Half Equation.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine To Chloride Ions Half Equation if we check the charge on both sides of the equation, we see they are the same—2+. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. half equations can be used to represent these reactions (higher tier): The slideshow shows what happens when. The reaction between chlorine and iron. Chloride ions from a chlorine molecule:. each chlorine. Chlorine To Chloride Ions Half Equation.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine To Chloride Ions Half Equation Chloride ions from a chlorine molecule:. half equations can be used to represent these reactions (higher tier): (in reality, this positive charge is. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: The reaction between chlorine and. Chlorine To Chloride Ions Half Equation.
From hxewfgvpo.blob.core.windows.net
Chlorine Ionic Reaction at Howard Saad blog Chlorine To Chloride Ions Half Equation (in reality, this positive charge is. In the process, the chlorine is reduced to chloride ions. The slideshow shows what happens when. if we check the charge on both sides of the equation, we see they are the same—2+. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and. Chlorine To Chloride Ions Half Equation.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation, free Chlorine To Chloride Ions Half Equation chlorine gas oxidizes iron(ii) ions to iron(iii) ions. The reaction between chlorine and iron. half equations can be used to represent these reactions (higher tier): each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: (in reality, this positive charge is. The slideshow shows what happens. Chlorine To Chloride Ions Half Equation.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine To Chloride Ions Half Equation half equations can be used to represent these reactions (higher tier): The slideshow shows what happens when. if we check the charge on both sides of the equation, we see they are the same—2+. Chloride ions from a chlorine molecule:. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could. Chlorine To Chloride Ions Half Equation.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine To Chloride Ions Half Equation The slideshow shows what happens when. The reaction between chlorine and iron. Chloride ions from a chlorine molecule:. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. In the process, the chlorine is reduced to chloride ions. half equations can be used to represent these reactions (higher tier): cl 2 (aq) + 2ki (aq) → 2kcl (aq) +. Chlorine To Chloride Ions Half Equation.
From www.youtube.com
Revision chemistry half equation for the formation of chlorine from Chlorine To Chloride Ions Half Equation each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The slideshow shows what happens when. chlorine gas oxidizes iron(ii) ions to iron(iii) ions.. Chlorine To Chloride Ions Half Equation.
From www.snexplores.org
Explainer Ions and radicals in our world Chlorine To Chloride Ions Half Equation The slideshow shows what happens when. The reaction between chlorine and iron. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture. Chlorine To Chloride Ions Half Equation.
From www.doubtnut.com
In passing chlorine gas through a concentrated solution of alkali we g Chlorine To Chloride Ions Half Equation The reaction between chlorine and iron. if we check the charge on both sides of the equation, we see they are the same—2+. Chloride ions from a chlorine molecule:. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: chlorine gas oxidizes iron(ii) ions to iron(iii). Chlorine To Chloride Ions Half Equation.
From www.slideshare.net
Chemical Reactions Chlorine To Chloride Ions Half Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: The reaction between chlorine and iron. chlorine gas oxidizes iron(ii) ions to iron(iii) ions.. Chlorine To Chloride Ions Half Equation.
From www.elevise.co.uk
C4 L) Electrolysis Part 2 AQA Chemistry Elevise Chlorine To Chloride Ions Half Equation half equations can be used to represent these reactions (higher tier): if we check the charge on both sides of the equation, we see they are the same—2+. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. chlorine gas oxidizes iron(ii) ions to. Chlorine To Chloride Ions Half Equation.
From www.slideserve.com
PPT The Chemistry of Sodium Chloride PowerPoint Presentation, free Chlorine To Chloride Ions Half Equation The slideshow shows what happens when. if we check the charge on both sides of the equation, we see they are the same—2+. Chloride ions from a chlorine molecule:. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. (in reality, this positive charge is. In the process, the chlorine is reduced to chloride ions. each chlorine atom in. Chlorine To Chloride Ions Half Equation.
From www.slideshare.net
Gcse c5 electrolysis revison Chlorine To Chloride Ions Half Equation chlorine gas oxidizes iron(ii) ions to iron(iii) ions. In the process, the chlorine is reduced to chloride ions. half equations can be used to represent these reactions (higher tier): cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. (in reality, this positive charge is.. Chlorine To Chloride Ions Half Equation.
From fyogqonuf.blob.core.windows.net
Chlorine Salt Chemical Formula at Clarence McPherson blog Chlorine To Chloride Ions Half Equation each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: (in reality, this positive charge is. The slideshow shows what happens when. if we check the charge on both sides of the equation, we see they are the same—2+. Chloride ions from a chlorine molecule:. In the. Chlorine To Chloride Ions Half Equation.
From www.youtube.com
WHICH DOES NOT GIVE REDOX REACTIONHypochlorite ion,Chlorite ion Chlorine To Chloride Ions Half Equation each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: half equations can be used to represent these reactions (higher tier): In the process, the chlorine is reduced to chloride ions. if we check the charge on both sides of the equation, we see they are. Chlorine To Chloride Ions Half Equation.
From www.numerade.com
In a basic solution, molecular chlorine, Cl2, reacts with hydroxide Chlorine To Chloride Ions Half Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. half equations can be used to represent these reactions (higher tier): The reaction between chlorine and iron. chlorine gas oxidizes iron(ii) ions to iron(iii) ions. if we check the charge on both sides of. Chlorine To Chloride Ions Half Equation.
From slideplayer.com
Starter for ppt download Chlorine To Chloride Ions Half Equation each chlorine atom in a chlorine molecule gains an electron (it is being reduced), so you could write this half equation: In the process, the chlorine is reduced to chloride ions. The reaction between chlorine and iron. (in reality, this positive charge is. Chloride ions from a chlorine molecule:. half equations can be used to represent these reactions. Chlorine To Chloride Ions Half Equation.