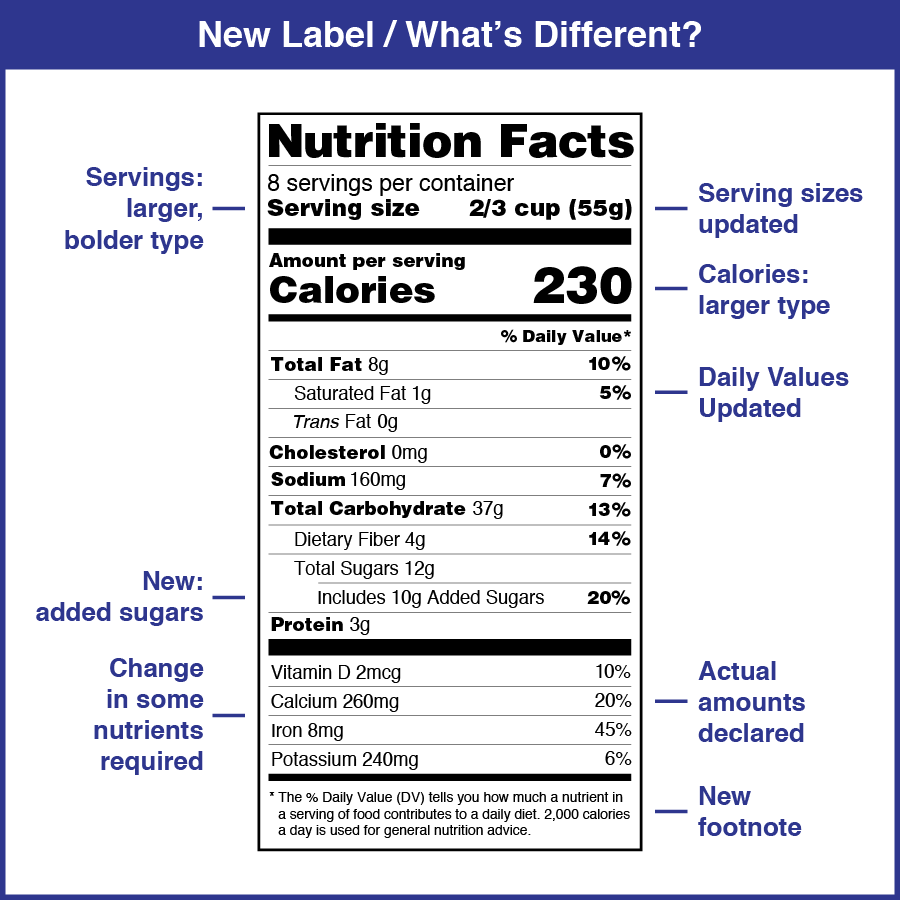
Fda Medication Guide Requirements . 208.20 content and format of a medication. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. under current regulations, fda requires a medication guide when fda determines one or more of the following. learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. subpart b —general requirements for a medication guide § 208.20 previous; currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for.
from www.fdalisting.com
subpart b —general requirements for a medication guide § 208.20 previous; learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. under current regulations, fda requires a medication guide when fda determines one or more of the following. 208.20 content and format of a medication. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for.
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements
Fda Medication Guide Requirements learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. 208.20 content and format of a medication. learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. under current regulations, fda requires a medication guide when fda determines one or more of the following. subpart b —general requirements for a medication guide § 208.20 previous;
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Guide Requirements currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not. Fda Medication Guide Requirements.
From www.slideserve.com
PPT Prescription Drug Information for Patients History PowerPoint Fda Medication Guide Requirements medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. 208.20. Fda Medication Guide Requirements.
From globalvision.co
Your Guide to Meeting FDA Labeling Requirements GlobalVision Fda Medication Guide Requirements currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. 208.20 content and format of a medication. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. learn about different types of patient labeling for prescription drugs,. Fda Medication Guide Requirements.
From ramtechno.com
What Are the Three FDA Classes for Medical Devices? RAM Technologies Fda Medication Guide Requirements the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. subpart b —general requirements for a medication guide § 208.20 previous; learn about different types of patient labeling for prescription. Fda Medication Guide Requirements.
From www.youtube.com
How to read a medication label YouTube Fda Medication Guide Requirements 208.20 content and format of a medication. under current regulations, fda requires a medication guide when fda determines one or more of the following. subpart b —general requirements for a medication guide § 208.20 previous; currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. learn when and. Fda Medication Guide Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Medication Guide Requirements currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. 208.20. Fda Medication Guide Requirements.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Medication Guide Requirements medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. subpart b —general requirements for a medication guide § 208.20 previous; under current regulations, fda requires a medication. Fda Medication Guide Requirements.
From www.researchgate.net
Example medication guide distributed to patients per United States Food Fda Medication Guide Requirements currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. under current regulations, fda requires a medication guide when fda determines one or more of the following. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a.. Fda Medication Guide Requirements.
From dimensionsofdentalhygiene.com
FDA Releases New Guidelines for Medication Labeling Fda Medication Guide Requirements learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. 208.20 content and format of a medication. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. the fda requires patient labeling for human prescription drug products that pose. Fda Medication Guide Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Medication Guide Requirements medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. 208.20 content and format of a medication. currently, fda requires that patients receive. Fda Medication Guide Requirements.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Medication Guide Requirements the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. subpart b —general requirements for a medication guide § 208.20 previous; learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. under current regulations, fda requires a medication guide when fda determines. Fda Medication Guide Requirements.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Medication Guide Requirements learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. under current regulations, fda requires a medication guide when fda determines one or. Fda Medication Guide Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Guide Requirements under current regulations, fda requires a medication guide when fda determines one or more of the following. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. 208.20 content and format of a medication. the fda requires patient labeling for human prescription drug products that pose a serious and. Fda Medication Guide Requirements.
From www.fdabasics.com
US FDA Registration is required for your product ? Understanding US FDA Fda Medication Guide Requirements under current regulations, fda requires a medication guide when fda determines one or more of the following. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary.. Fda Medication Guide Requirements.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Medication Guide Requirements subpart b —general requirements for a medication guide § 208.20 previous; under current regulations, fda requires a medication guide when fda determines one or more of the following. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. 208.20 content and format of a medication.. Fda Medication Guide Requirements.
From www.inspiremalibu.com
A Brief Guide to Drug Scheduling in the United States Fda Medication Guide Requirements learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. subpart b —general requirements for a medication guide § 208.20 previous; currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. learn about different types of. Fda Medication Guide Requirements.
From www.fda.gov
FDA Drug Approval Process Infographic (Horizontal) FDA Fda Medication Guide Requirements the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. 208.20 content and format of a medication. medication guide should be required as labeling but not part of a rems if fda determines. Fda Medication Guide Requirements.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Regulations Guide Artwork Flow Fda Medication Guide Requirements subpart b —general requirements for a medication guide § 208.20 previous; under current regulations, fda requires a medication guide when fda determines one or more of the following. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. 208.20 content and format of a medication.. Fda Medication Guide Requirements.
From simpleandpractical.com
FDAapproved 'Medication Guides' for patients Simple and Practical Fda Medication Guide Requirements currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. 208.20 content and format of a medication. under current regulations, fda requires a medication guide when fda determines one or more of the following. medication guide should be required as labeling but not part of a rems if fda. Fda Medication Guide Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Guide Requirements currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. subpart b —general requirements for a medication guide § 208.20 previous; under current regulations, fda requires. Fda Medication Guide Requirements.
From www.slideserve.com
PPT Medication Guides Update PowerPoint Presentation, free download Fda Medication Guide Requirements 208.20 content and format of a medication. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. medication guide should be required as labeling but not part of a rems if. Fda Medication Guide Requirements.
From dokumen.tips
(PDF) FDA Guidelines for Medication Guide Distribution …pharmacy.ca Fda Medication Guide Requirements the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. 208.20 content and format of a medication. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. learn when and how to distribute medication guides for prescription drugs and biologics, and. Fda Medication Guide Requirements.
From deal.town
Medication Guide 6/27/2023 US FDA Fda Medication Guide Requirements learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. under current regulations, fda requires a medication guide when fda determines one or more of the following. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. currently,. Fda Medication Guide Requirements.
From www.greenlight.guru
FDA Labeling Requirements Checklist Free Download Fda Medication Guide Requirements learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. . Fda Medication Guide Requirements.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Medication Guide Requirements learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. . Fda Medication Guide Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Medication Guide Requirements learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. subpart b —general requirements for a medication guide § 208.20 previous; currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. under current regulations, fda requires a medication guide when fda determines. Fda Medication Guide Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Guide Requirements the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of. Fda Medication Guide Requirements.
From www.i3cglobal.com
FDA Labelling Requirements Guidance and Pricing Fda Medication Guide Requirements learn about different types of patient labeling for prescription drugs, such as instructions for use, medication. under current regulations, fda requires a medication guide when fda determines one or more of the following. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. subpart b —general requirements for. Fda Medication Guide Requirements.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Fda Medication Guide Requirements learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. under current regulations, fda requires a medication guide when fda determines one or more of the following. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for.. Fda Medication Guide Requirements.
From www.slideserve.com
PPT Labeling Prescription Drugs for Physicians and Consumers (FDA Fda Medication Guide Requirements learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. under current regulations, fda requires a medication guide when fda determines one or more of the following. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for.. Fda Medication Guide Requirements.
From deal.town
Medication Guide 4/21/2023 US FDA Fda Medication Guide Requirements under current regulations, fda requires a medication guide when fda determines one or more of the following. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a.. Fda Medication Guide Requirements.
From www.fda.gov
Patient Labeling Resources FDA Fda Medication Guide Requirements the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. under current regulations, fda requires a medication guide when fda determines one or more of the following. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a.. Fda Medication Guide Requirements.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Medication Guide Requirements under current regulations, fda requires a medication guide when fda determines one or more of the following. learn when and how to distribute medication guides for prescription drugs and biologics, and when they are required as part of a. currently, fda requires that patients receive information about select prescription drugs in the form of medication guides for.. Fda Medication Guide Requirements.
From www.slideserve.com
PPT Prescription Drug Information for Patients History PowerPoint Fda Medication Guide Requirements 208.20 content and format of a medication. under current regulations, fda requires a medication guide when fda determines one or more of the following. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. learn about different types of patient labeling for prescription drugs, such. Fda Medication Guide Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Fda Medication Guide Requirements the fda requires patient labeling for human prescription drug products that pose a serious and significant public health. 208.20 content and format of a medication. medication guide should be required as labeling but not part of a rems if fda determines that a rems is not necessary. learn when and how to distribute medication guides for prescription. Fda Medication Guide Requirements.