What Is A Sharp Melting Point . melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The transition between the solid and the liquid is so. In contrast, amorphous solids have irregular or curved. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. a student tests the melting point of a sample of sulfur. As heat is applied to a solid, its temperature will increase. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting.
from www.mdpi.com
pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. a student tests the melting point of a sample of sulfur. In contrast, amorphous solids have irregular or curved. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. As heat is applied to a solid, its temperature will increase. The transition between the solid and the liquid is so.
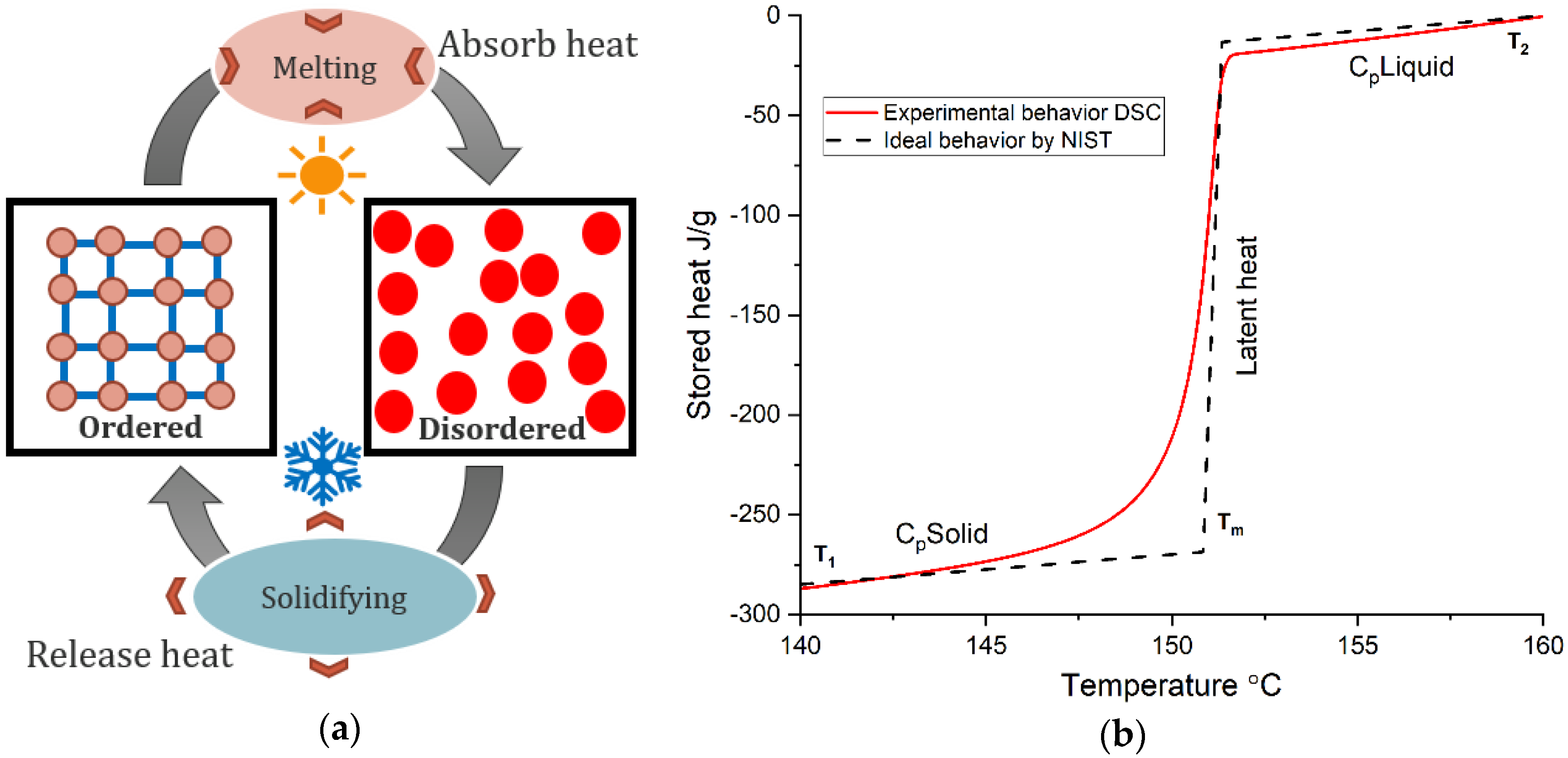
Applied Sciences Free FullText Thermal Characterization of Phase
What Is A Sharp Melting Point pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. In contrast, amorphous solids have irregular or curved. The transition between the solid and the liquid is so. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. As heat is applied to a solid, its temperature will increase. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. a student tests the melting point of a sample of sulfur. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid.
From www.coursehero.com
[Solved] What are the melting point characteristics of a pure compound What Is A Sharp Melting Point pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. In contrast, amorphous solids have irregular or curved. a student tests the melting point of a sample of sulfur. . What Is A Sharp Melting Point.
From www.youtube.com
Melting Point Determination with MelTemp YouTube What Is A Sharp Melting Point pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. a student tests the melting point of a sample of sulfur. melting point, temperature at which the solid. What Is A Sharp Melting Point.
From quizdbcornwallis.z21.web.core.windows.net
What Is Iron's Melting Point What Is A Sharp Melting Point a student tests the melting point of a sample of sulfur. The transition between the solid and the liquid is so. As heat is applied to a solid, its temperature will increase. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. In contrast, amorphous solids have irregular or. What Is A Sharp Melting Point.
From www.slideserve.com
PPT Theory of Melting Point PowerPoint Presentation, free download What Is A Sharp Melting Point pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. As heat is applied to a solid, its temperature will increase. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. pure substances have sharp, well defined melting. What Is A Sharp Melting Point.
From www.numerade.com
SOLVED Pure R,Rhydrobenzoin (a side product of mesohydrobenzoin) has What Is A Sharp Melting Point pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. The transition between the solid and the liquid is so. a student tests the melting point of a sample of sulfur. in this section is described the theory behind the phenomenon of melting point depression (which is identical. What Is A Sharp Melting Point.
From www.toppr.com
Which of the following polymers shows a sharp melting point? What Is A Sharp Melting Point a student tests the melting point of a sample of sulfur. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. In contrast, amorphous solids have irregular or curved. . What Is A Sharp Melting Point.
From www.chegg.com
2. Purpose of measuring melting point, using simple What Is A Sharp Melting Point In contrast, amorphous solids have irregular or curved. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. The transition between the solid and the liquid is so. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure substances. What Is A Sharp Melting Point.
From studylib.net
Exp 1 Melting Point, Boiling Point, and Index of Refraction What Is A Sharp Melting Point In contrast, amorphous solids have irregular or curved. The transition between the solid and the liquid is so. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. a student tests the melting point of a sample of sulfur. in this section is described the theory behind the. What Is A Sharp Melting Point.
From www.youtube.com
Difference between Melting point / Boiling point & Freezing point What Is A Sharp Melting Point The transition between the solid and the liquid is so. a student tests the melting point of a sample of sulfur. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. In contrast, amorphous solids have irregular or curved. pure, crystalline solids have a characteristic melting point,. What Is A Sharp Melting Point.
From www.slideserve.com
PPT Degradation & Stabilization of Polymers PowerPoint Presentation What Is A Sharp Melting Point melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. a student tests the melting point of a sample of sulfur. pure substances have sharp, well defined melting. What Is A Sharp Melting Point.
From www.slideserve.com
PPT PHYSICAL PROPERTIES OF ORGANIC COMPOUNDS PowerPoint Presentation What Is A Sharp Melting Point in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium.. What Is A Sharp Melting Point.
From www.numerade.com
SOLVED explain why polymers melt over a range of temperatures unlike What Is A Sharp Melting Point pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. The transition between the solid and the liquid is so. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. As heat is applied to a solid, its temperature will. What Is A Sharp Melting Point.
From sciencenotes.org
Melting Point Definition and List What Is A Sharp Melting Point The transition between the solid and the liquid is so. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. in this section is described the theory behind the phenomenon. What Is A Sharp Melting Point.
From www.knowledgeboat.com
The melting point of naphthalene is 80°C and the room KnowledgeBoat What Is A Sharp Melting Point melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The transition between the solid and the liquid is so. a student tests the melting point of a sample of sulfur. in this section is described the theory behind the phenomenon of melting point depression (which is identical to. What Is A Sharp Melting Point.
From ericvisser.nl
Melting point chart Ericvisser What Is A Sharp Melting Point The transition between the solid and the liquid is so. In contrast, amorphous solids have irregular or curved. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. As heat is applied to a solid, its temperature will increase. melting point, temperature at which the solid and liquid. What Is A Sharp Melting Point.
From www.chegg.com
Solved 1. When doing a mixed melting point determination, What Is A Sharp Melting Point As heat is applied to a solid, its temperature will increase. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. The transition between the solid and the liquid is so.. What Is A Sharp Melting Point.
From eduinput.com
7 Difference between crystalline solids and amorphous solids What Is A Sharp Melting Point pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. a student tests the melting point of a sample of sulfur. in this section is described the theory. What Is A Sharp Melting Point.
From www.studocu.com
Determination of Melting Point 2 pure organic compounds usually have What Is A Sharp Melting Point in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. a student tests the melting point of a sample of sulfur. The transition between the solid and the liquid. What Is A Sharp Melting Point.
From www.tainstruments.com
“Apparent Melting” A New Approach to Characterizing Crystalline What Is A Sharp Melting Point As heat is applied to a solid, its temperature will increase. a student tests the melting point of a sample of sulfur. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. melting point, temperature at which the solid and liquid forms of a pure substance can exist. What Is A Sharp Melting Point.
From www.slideserve.com
PPT Melting Point and Boiling Point PowerPoint Presentation, free What Is A Sharp Melting Point The transition between the solid and the liquid is so. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. a student tests the melting point of a sample of sulfur. In contrast, amorphous solids have irregular or curved. As heat is applied to a solid, its temperature will increase.. What Is A Sharp Melting Point.
From thechemistrynotes.com
Crystallography Crystals and laws of crystallography What Is A Sharp Melting Point melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The transition between the solid and the liquid is so. In contrast, amorphous solids have irregular or curved. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. a student. What Is A Sharp Melting Point.
From www.researchgate.net
DSC traces of [1Me2NO 23HIM][Pic] (8a) showing a sharp melting What Is A Sharp Melting Point The transition between the solid and the liquid is so. As heat is applied to a solid, its temperature will increase. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing. What Is A Sharp Melting Point.
From studylib.net
Melting Points and Mixed Melting Points What Is A Sharp Melting Point a student tests the melting point of a sample of sulfur. As heat is applied to a solid, its temperature will increase. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. in this section is described the theory behind the phenomenon of melting point depression (which is. What Is A Sharp Melting Point.
From www.worksheetsplanet.com
What is Melting Point Definition of Melting Point What Is A Sharp Melting Point melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. As heat is applied to a solid, its temperature will increase. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. pure substances have sharp, well defined melting points, whereas. What Is A Sharp Melting Point.
From www.youtube.com
How to determine the Melting Point of a Substance? Experiment YouTube What Is A Sharp Melting Point In contrast, amorphous solids have irregular or curved. The transition between the solid and the liquid is so. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure. What Is A Sharp Melting Point.
From www.studocu.com
Melting Points Gizmo Name Date Student Exploration Melting Points What Is A Sharp Melting Point pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. The transition between the solid and the liquid is so. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. a student tests the melting point of a sample of. What Is A Sharp Melting Point.
From www.thoughtco.com
What Is the Definition of Melting Point? What Is A Sharp Melting Point melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. As heat is applied to a solid, its temperature will increase. pure, crystalline solids have a characteristic melting point,. What Is A Sharp Melting Point.
From www.chegg.com
Solved EXPERIMENT 1 Melting Point and Mixed Melting Point What Is A Sharp Melting Point a student tests the melting point of a sample of sulfur. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The transition between the solid and the liquid is so. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a. What Is A Sharp Melting Point.
From www.mdpi.com
Applied Sciences Free FullText Thermal Characterization of Phase What Is A Sharp Melting Point The transition between the solid and the liquid is so. a student tests the melting point of a sample of sulfur. As heat is applied to a solid, its temperature will increase. In contrast, amorphous solids have irregular or curved. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium.. What Is A Sharp Melting Point.
From www.slideserve.com
PPT Melting Point & Refractive Index PowerPoint Presentation ID6266260 What Is A Sharp Melting Point As heat is applied to a solid, its temperature will increase. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The transition between the solid and the liquid is. What Is A Sharp Melting Point.
From www.youtube.com
Determination of Melting Point YouTube What Is A Sharp Melting Point In contrast, amorphous solids have irregular or curved. The transition between the solid and the liquid is so. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. As heat is. What Is A Sharp Melting Point.
From www.slideserve.com
PPT Melting Point & Refractive Index PowerPoint Presentation ID6266260 What Is A Sharp Melting Point pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. As. What Is A Sharp Melting Point.
From cedqzwdi.blob.core.windows.net
What Is The Melting Point Of Snow at Arthur Taylor blog What Is A Sharp Melting Point melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. in this section is described the theory behind the phenomenon of melting point depression (which is identical to freezing point. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid.. What Is A Sharp Melting Point.
From www.differencebetween.com
Difference Between Melting Point and Freezing Point Compare the What Is A Sharp Melting Point a student tests the melting point of a sample of sulfur. pure substances have sharp, well defined melting points, whereas the addition of impurity both lowers and broadens the melting. pure, crystalline solids have a characteristic melting point, the temperature at which the solid melts to become a liquid. The transition between the solid and the liquid. What Is A Sharp Melting Point.
From www.slideserve.com
PPT PHYSICAL PROPERTIES OF ORGANIC COMPOUNDS PowerPoint Presentation What Is A Sharp Melting Point In contrast, amorphous solids have irregular or curved. As heat is applied to a solid, its temperature will increase. The transition between the solid and the liquid is so. melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. pure, crystalline solids have a characteristic melting point, the temperature at. What Is A Sharp Melting Point.