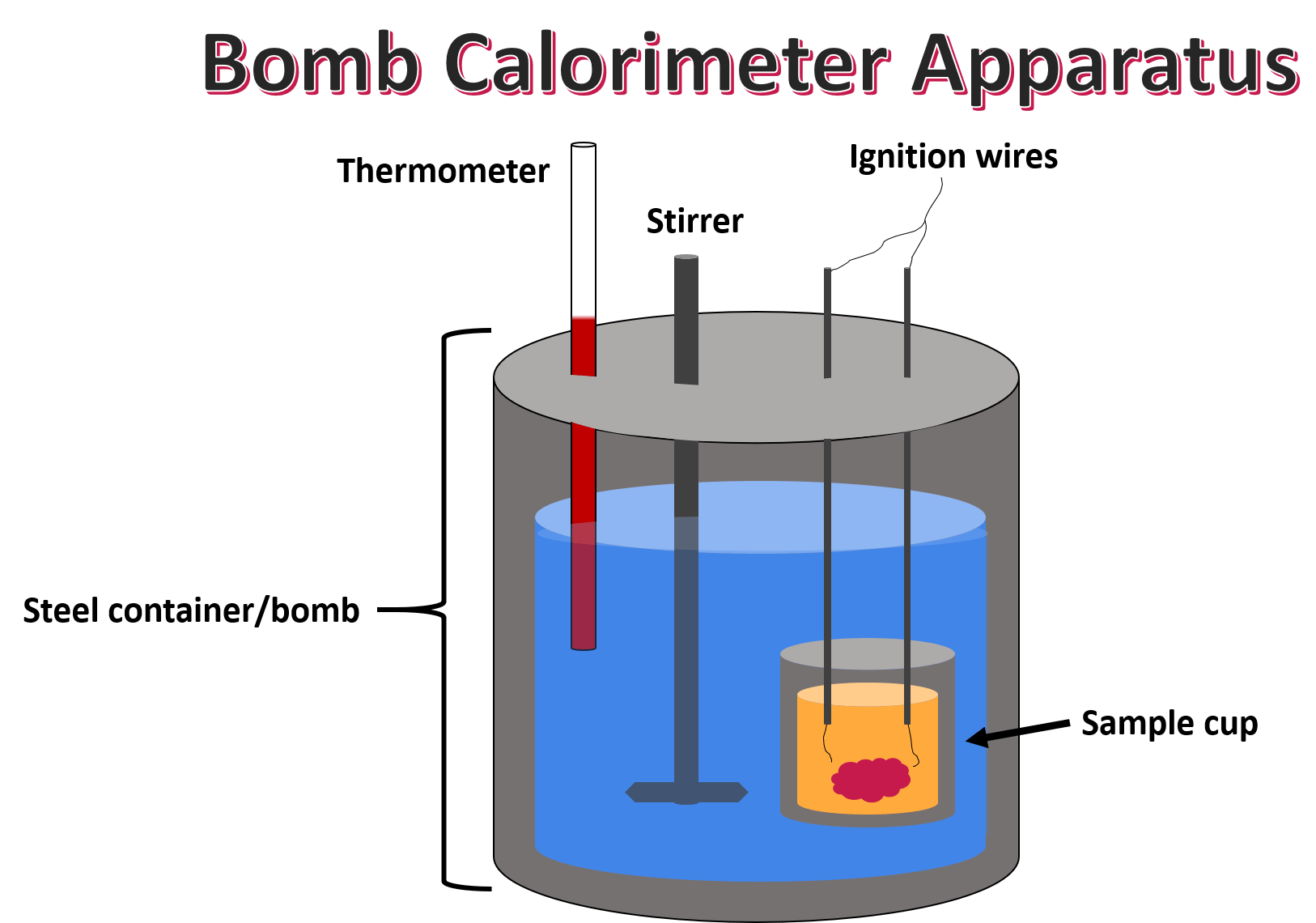
What Would Make An Appropriate Calorimeter . apply the first law of thermodynamics to calorimetry. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. Measuring heat flow is essential in numerous scientific, engineering,. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is a calorimeter?
from www.expii.com
apply the first law of thermodynamics to calorimetry. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. what is a calorimeter?
Bomb Calorimeter — Structure & Function Expii
What Would Make An Appropriate Calorimeter Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is a calorimeter? Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Measuring heat flow is essential in numerous scientific, engineering,. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. apply the first law of thermodynamics to calorimetry.
From rachaelocean.blogspot.com
32+ calorimeter constant calculator RachaelOcean What Would Make An Appropriate Calorimeter a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. apply the first law of thermodynamics to calorimetry. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. a calorimeter is a device used to measure the amount of heat. What Would Make An Appropriate Calorimeter.
From www.shemmassianconsulting.com
Thermochemistry for the MCAT Everything You Need to Know — Shemmassian What Would Make An Appropriate Calorimeter they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. what is a calorimeter? Laboratory calorimeters measure the enthalpy, or heat transfer. What Would Make An Appropriate Calorimeter.
From chem.libretexts.org
6.5 Constant Volume Calorimetry Measuring ΔU for Chemical Reactions What Would Make An Appropriate Calorimeter they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or. What Would Make An Appropriate Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Would Make An Appropriate Calorimeter apply the first law of thermodynamics to calorimetry. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. what is a calorimeter? they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of. What Would Make An Appropriate Calorimeter.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube What Would Make An Appropriate Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Measuring heat flow is essential in numerous scientific, engineering,. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by.. What Would Make An Appropriate Calorimeter.
From huksefluxusa.com
What is a Calorimeter? — HuksefluxUSA What Would Make An Appropriate Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. a calorimeter is a device which is in use for measuring the warmth of. What Would Make An Appropriate Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Would Make An Appropriate Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters. What Would Make An Appropriate Calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Would Make An Appropriate Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is a calorimeter? a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Measuring heat flow is essential in numerous scientific, engineering,. they allow scientists. What Would Make An Appropriate Calorimeter.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper What Would Make An Appropriate Calorimeter apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Compare heat flow from hot to cold objects in. What Would Make An Appropriate Calorimeter.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure What Would Make An Appropriate Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. what is a calorimeter? Measuring heat flow is essential in numerous scientific, engineering,. Laboratory. What Would Make An Appropriate Calorimeter.
From users.highland.edu
Calorimetry What Would Make An Appropriate Calorimeter Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. apply the first law of thermodynamics to calorimetry. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. a calorimeter is a device used to measure the amount. What Would Make An Appropriate Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Would Make An Appropriate Calorimeter Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes. What Would Make An Appropriate Calorimeter.
From slideplayer.com
How a Particle Detector works ppt download What Would Make An Appropriate Calorimeter apply the first law of thermodynamics to calorimetry. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Measuring heat flow is essential in numerous scientific, engineering,. calorimeters are designed to minimize energy exchange between the system. What Would Make An Appropriate Calorimeter.
From www.scribd.com
GC2 Thermodynamics and Calorimetry PDF Heat Calorimetry What Would Make An Appropriate Calorimeter a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and. What Would Make An Appropriate Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. what is a calorimeter? apply the first law of thermodynamics to calorimetry. calorimeters are designed to minimize energy exchange between the system being studied and its. What Would Make An Appropriate Calorimeter.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Measuring heat flow. What Would Make An Appropriate Calorimeter.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. apply the first law of thermodynamics to calorimetry. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. what is a calorimeter? Measuring heat flow is essential in numerous scientific, engineering,. they allow scientists to determine. What Would Make An Appropriate Calorimeter.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter What Would Make An Appropriate Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. what is a calorimeter? Measuring heat flow. What Would Make An Appropriate Calorimeter.
From www.animalia-life.club
Calorimeter Diagram What Would Make An Appropriate Calorimeter calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Measuring heat flow is essential in numerous scientific, engineering,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. what is a calorimeter? Compare. What Would Make An Appropriate Calorimeter.
From www.chegg.com
Solved Use the References to access important values if What Would Make An Appropriate Calorimeter calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. what is a calorimeter? apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. they allow scientists to determine the amount of heat energy involved in a. What Would Make An Appropriate Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Would Make An Appropriate Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Measuring heat. What Would Make An Appropriate Calorimeter.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow. What Would Make An Appropriate Calorimeter.
From www.savemyexams.com
The Transfer of Biomass OCR A Level Biology Revision Notes 2017 What Would Make An Appropriate Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and. What Would Make An Appropriate Calorimeter.
From www.huck.psu.edu
Isothermal Titration Calorimetry Automated Calorimetry Facility The What Would Make An Appropriate Calorimeter Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. they allow scientists to determine the amount of heat energy involved in a reaction, which. What Would Make An Appropriate Calorimeter.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization. What Would Make An Appropriate Calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Would Make An Appropriate Calorimeter calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. What Would Make An Appropriate Calorimeter.
From www.researchgate.net
Diagram of drop calorimeter. Download Scientific Diagram What Would Make An Appropriate Calorimeter Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Measuring heat flow is essential in numerous scientific, engineering,. a calorimeter is a device which is in use for measuring the warmth of. What Would Make An Appropriate Calorimeter.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry What Would Make An Appropriate Calorimeter a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes also as. Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device used to measure the amount of heat involved. What Would Make An Appropriate Calorimeter.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry What Would Make An Appropriate Calorimeter Measuring heat flow is essential in numerous scientific, engineering,. Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device which is in use for. What Would Make An Appropriate Calorimeter.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses What Would Make An Appropriate Calorimeter they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Laboratory calorimeters measure. What Would Make An Appropriate Calorimeter.
From www.education.com
Calorimetry Bomb Calorimeter Experiment What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters are designed to minimize energy exchange between. What Would Make An Appropriate Calorimeter.
From evulpo.com
evulpo Calorimetry What Would Make An Appropriate Calorimeter what is a calorimeter? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. calorimeters are designed to minimize energy exchange between the. What Would Make An Appropriate Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is a calorimeter? a calorimeter is a device which is in use for measuring the warmth of chemical reactions or physical changes. What Would Make An Appropriate Calorimeter.
From slideplayer.com
A “Calorimeter”. Calorimetry Calculations When analyzing data obtained What Would Make An Appropriate Calorimeter Laboratory calorimeters measure the enthalpy, or heat transfer under constant pressure, produced or absorbed by. Measuring heat flow is essential in numerous scientific, engineering,. they allow scientists to determine the amount of heat energy involved in a reaction, which can help in the design and optimization of chemical. Compare heat flow from hot to cold objects in an ideal. What Would Make An Appropriate Calorimeter.
From www.thetechedvocate.org
How to Build a Calorimeter The Tech Edvocate What Would Make An Appropriate Calorimeter Measuring heat flow is essential in numerous scientific, engineering,. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeters are designed to minimize energy exchange between the system being studied and its surroundings. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. What Would Make An Appropriate Calorimeter.