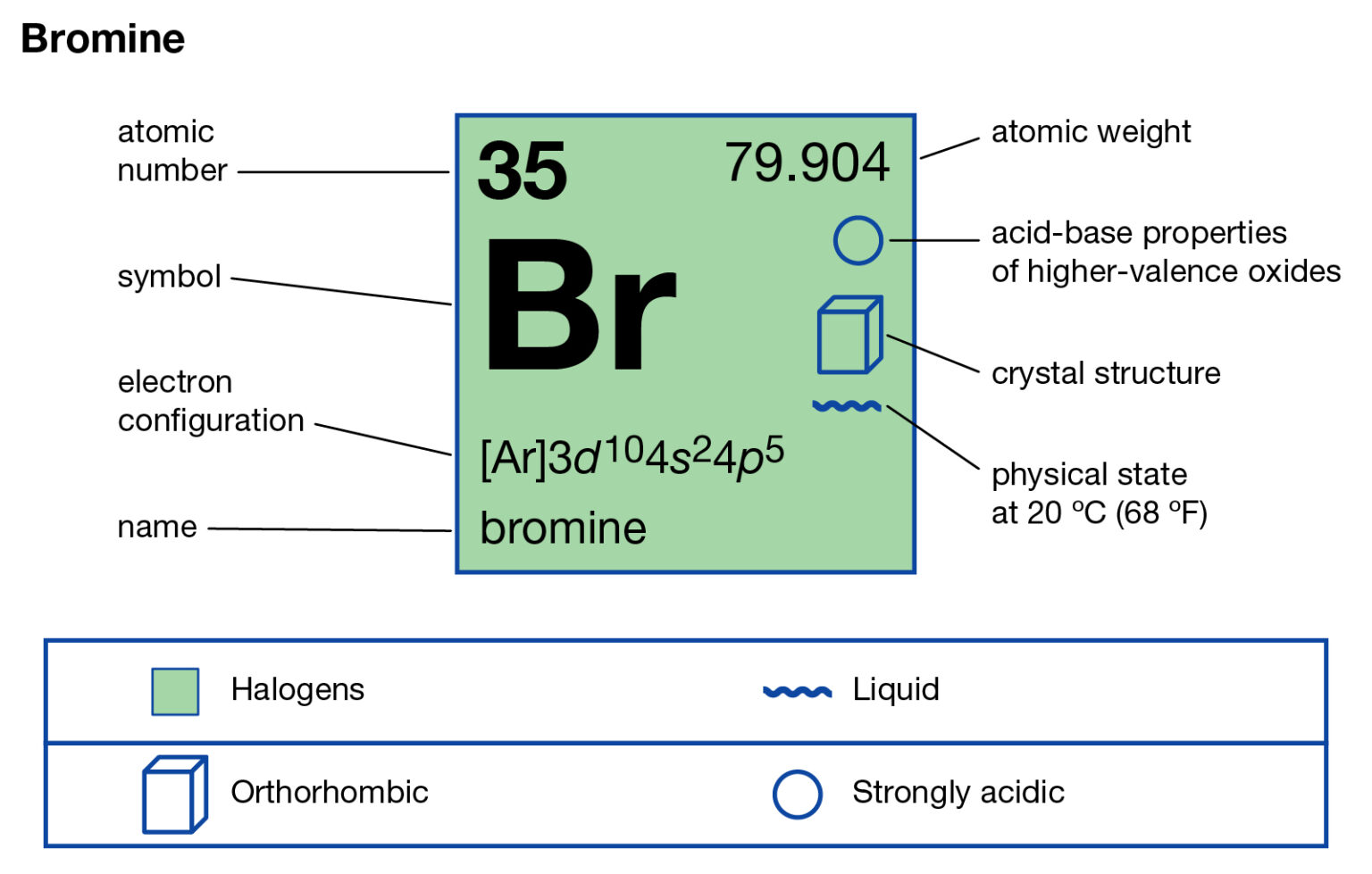
When Bromine (Br) Becomes An Ion It Will Become A . Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Atoms of elements gain or lose electron (s) to attain a. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. An atom of bromine (br) forms an ion and becomes br⁻. Here br− ion is stable due to noble gas. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on.
from mavink.com
Atoms of group 17 gain one electron and form anions with a 1− charge; A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of elements gain or lose electron (s) to attain a. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Here br− ion is stable due to noble gas. An atom of bromine (br) forms an ion and becomes br⁻. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive.
Lewis Dot Diagram For Bromine
When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Here br− ion is stable due to noble gas. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Atoms of elements gain or lose electron (s) to attain a. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. An atom of bromine (br) forms an ion and becomes br⁻.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. An atom of bromine (br) forms an ion and. When Bromine (Br) Becomes An Ion It Will Become A.
From proper-cooking.info
Bromine Atomic Structure When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Here br− ion is stable due to noble gas. Atoms of elements gain or lose electron (s) to attain a. An atom of bromine (br) forms an ion and becomes br⁻. A bromine ion, denoted as br −, is the common negative univalent. When Bromine (Br) Becomes An Ion It Will Become A.
From mavink.com
Lewis Dot Diagram For Bromine When Bromine (Br) Becomes An Ion It Will Become A Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. An atom of bromine (br) forms an ion and becomes br⁻. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. A bromine. When Bromine (Br) Becomes An Ion It Will Become A.
From www.numerade.com
SOLVED 'Write the abbreviated electron configuration for bromine' When Bromine (Br) Becomes An Ion It Will Become A Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms. When Bromine (Br) Becomes An Ion It Will Become A.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Properties, Structure, Facts When Bromine (Br) Becomes An Ion It Will Become A Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Atoms of group 17 gain one electron and form anions with a 1− charge; In this article, you will learn about the ionic charge,. When Bromine (Br) Becomes An Ion It Will Become A.
From www.pinterest.com
Bromine Facts Atomic Number 35 and Element Symbol Br When Bromine (Br) Becomes An Ion It Will Become A A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. An atom of bromine (br) forms an ion and becomes br⁻. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Bromine molecule undergo heterolytic cleavage to form br+ and. When Bromine (Br) Becomes An Ion It Will Become A.
From www.animalia-life.club
Electron Configuration For Bromine When Bromine (Br) Becomes An Ion It Will Become A Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Atoms of elements gain or lose electron (s) to attain a. Here br− ion is stable due to noble gas. An atom of bromine (br) forms an ion and becomes br⁻. Atoms of group 16 gain two electrons and. When Bromine (Br) Becomes An Ion It Will Become A.
From www.youtube.com
How to Draw the Lewis Dot Structure for Br (Bromide ion) YouTube When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Here br− ion is stable due to noble gas. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of. When Bromine (Br) Becomes An Ion It Will Become A.
From www.slideserve.com
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529 When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 17 gain one electron and form anions with a 1− charge; In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Here br− ion is stable due to noble gas. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Bromine naturally forms an anion, a negatively charged ion,. When Bromine (Br) Becomes An Ion It Will Become A.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy When Bromine (Br) Becomes An Ion It Will Become A Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of elements gain or lose electron (s) to attain a. Here br− ion is stable due to noble gas. An atom. When Bromine (Br) Becomes An Ion It Will Become A.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy When Bromine (Br) Becomes An Ion It Will Become A In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Atoms of elements gain or lose electron (s) to attain a. Here br− ion is stable due to noble gas. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Bromine molecule undergo heterolytic cleavage to. When Bromine (Br) Becomes An Ion It Will Become A.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. When Bromine (Br) Becomes An Ion It Will Become A.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube When Bromine (Br) Becomes An Ion It Will Become A An atom of bromine (br) forms an ion and becomes br⁻. Atoms of group 17 gain one electron and form anions with a 1− charge; Here br− ion is stable due to noble gas. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms that lose. When Bromine (Br) Becomes An Ion It Will Become A.
From cednwvxm.blob.core.windows.net
Bromine Formula Of Compound at Anthony Ford blog When Bromine (Br) Becomes An Ion It Will Become A An atom of bromine (br) forms an ion and becomes br⁻. Here br− ion is stable due to noble gas. Atoms of elements gain or lose electron (s) to attain a. Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms that lose electrons acquire a positive charge because they are left with fewer negatively. When Bromine (Br) Becomes An Ion It Will Become A.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine When Bromine (Br) Becomes An Ion It Will Become A Atoms of elements gain or lose electron (s) to attain a. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. An atom of bromine (br) forms an ion and becomes br⁻. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water. When Bromine (Br) Becomes An Ion It Will Become A.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 17 gain one electron and form anions with a 1− charge; Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. An atom of bromine (br) forms an ion and becomes br⁻. Bromine molecule undergo. When Bromine (Br) Becomes An Ion It Will Become A.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube When Bromine (Br) Becomes An Ion It Will Become A In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. An atom of bromine (br) forms an ion and becomes br⁻. Atoms of group 17 gain one electron and form. When Bromine (Br) Becomes An Ion It Will Become A.
From www.chegg.com
Solved The correct mechanism for the addition of bromine to When Bromine (Br) Becomes An Ion It Will Become A Here br− ion is stable due to noble gas. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. An atom of bromine (br) forms an ion and becomes br⁻. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Bromine naturally forms an anion, a. When Bromine (Br) Becomes An Ion It Will Become A.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity When Bromine (Br) Becomes An Ion It Will Become A In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Here br− ion is stable. When Bromine (Br) Becomes An Ion It Will Become A.
From www.coursehero.com
[Solved] Review the videos, and then identify the bromine (Br Br) atoms in... Course Hero When Bromine (Br) Becomes An Ion It Will Become A Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Here br− ion is stable due to noble gas. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. An atom. When Bromine (Br) Becomes An Ion It Will Become A.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of group 17 gain one electron and form anions with a 1− charge; In this article, you will learn. When Bromine (Br) Becomes An Ion It Will Become A.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) When Bromine (Br) Becomes An Ion It Will Become A Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Here br− ion is stable due to noble gas. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. In this article, you will learn about the. When Bromine (Br) Becomes An Ion It Will Become A.
From joiglahlj.blob.core.windows.net
Electron Configuration Of Bromine at Johanna Brewton blog When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 17 gain one electron and form anions with a 1− charge; A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Bromine naturally forms an anion, a. When Bromine (Br) Becomes An Ion It Will Become A.
From www.youtube.com
Ionic Charge for Bromine (Br) YouTube When Bromine (Br) Becomes An Ion It Will Become A Here br− ion is stable due to noble gas. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of group 16 gain two electrons and form ions with a 2−. When Bromine (Br) Becomes An Ion It Will Become A.
From h-o-m-e.org
Bromide (Br) Ion Charges Explained When Bromine (Br) Becomes An Ion It Will Become A A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Atoms of elements gain or lose electron (s) to attain a. Here br− ion is stable due to noble gas. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine.. When Bromine (Br) Becomes An Ion It Will Become A.
From scienceinfo.com
Bromine (Br) Element Properties and Amazing Uses, Facts When Bromine (Br) Becomes An Ion It Will Become A Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A bromine ion, denoted as br −, is the common negative. When Bromine (Br) Becomes An Ion It Will Become A.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Table of the Elements When Bromine (Br) Becomes An Ion It Will Become A Here br− ion is stable due to noble gas. Atoms of group 17 gain one electron and form anions with a 1− charge; Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Atoms that lose electrons acquire a positive charge because. When Bromine (Br) Becomes An Ion It Will Become A.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 17 gain one electron and form anions with a 1− charge; Atoms of elements gain or lose electron (s) to attain a. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. A bromine ion, denoted as br −,. When Bromine (Br) Becomes An Ion It Will Become A.
From www.numerade.com
SOLVED How does an atom of bromine79 a bromide ion with a 1 charge? When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 17 gain one electron and form anions with a 1− charge; A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Atoms that lose electrons acquire a. When Bromine (Br) Becomes An Ion It Will Become A.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 17 gain one electron and form anions with a 1− charge; Here br− ion is stable due to noble gas. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. In this article, you will learn about the ionic charge, nuclear charge, zeff, and reactions of bromine. Bromine molecule undergo. When Bromine (Br) Becomes An Ion It Will Become A.
From www.youtube.com
Br electronic configurationHow to write electronic configuration of Bromine YouTube When Bromine (Br) Becomes An Ion It Will Become A Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Atoms of elements gain or lose electron (s) to attain a. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Atoms that lose electrons acquire a. When Bromine (Br) Becomes An Ion It Will Become A.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds, Reactivity When Bromine (Br) Becomes An Ion It Will Become A Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Here br− ion is stable due to noble gas. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Atoms of. When Bromine (Br) Becomes An Ion It Will Become A.
From www.numerade.com
SOLVED 23. What happens when a bromine atom a bromide ion? (A) A positive ion is formed When Bromine (Br) Becomes An Ion It Will Become A An atom of bromine (br) forms an ion and becomes br⁻. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is typically analyzed as water soluble bromine. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Atoms that lose electrons acquire a positive charge because they are. When Bromine (Br) Becomes An Ion It Will Become A.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Vector Image & Art Alamy When Bromine (Br) Becomes An Ion It Will Become A Atoms of elements gain or lose electron (s) to attain a. Bromine molecule undergo heterolytic cleavage to form br+ and br− ions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Atoms of. When Bromine (Br) Becomes An Ion It Will Become A.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram When Bromine (Br) Becomes An Ion It Will Become A An atom of bromine (br) forms an ion and becomes br⁻. Bromine naturally forms an anion, a negatively charged ion, when it gets into chemical reactions. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. A bromine ion, denoted as br −, is the common negative univalent form of bromine that is. When Bromine (Br) Becomes An Ion It Will Become A.