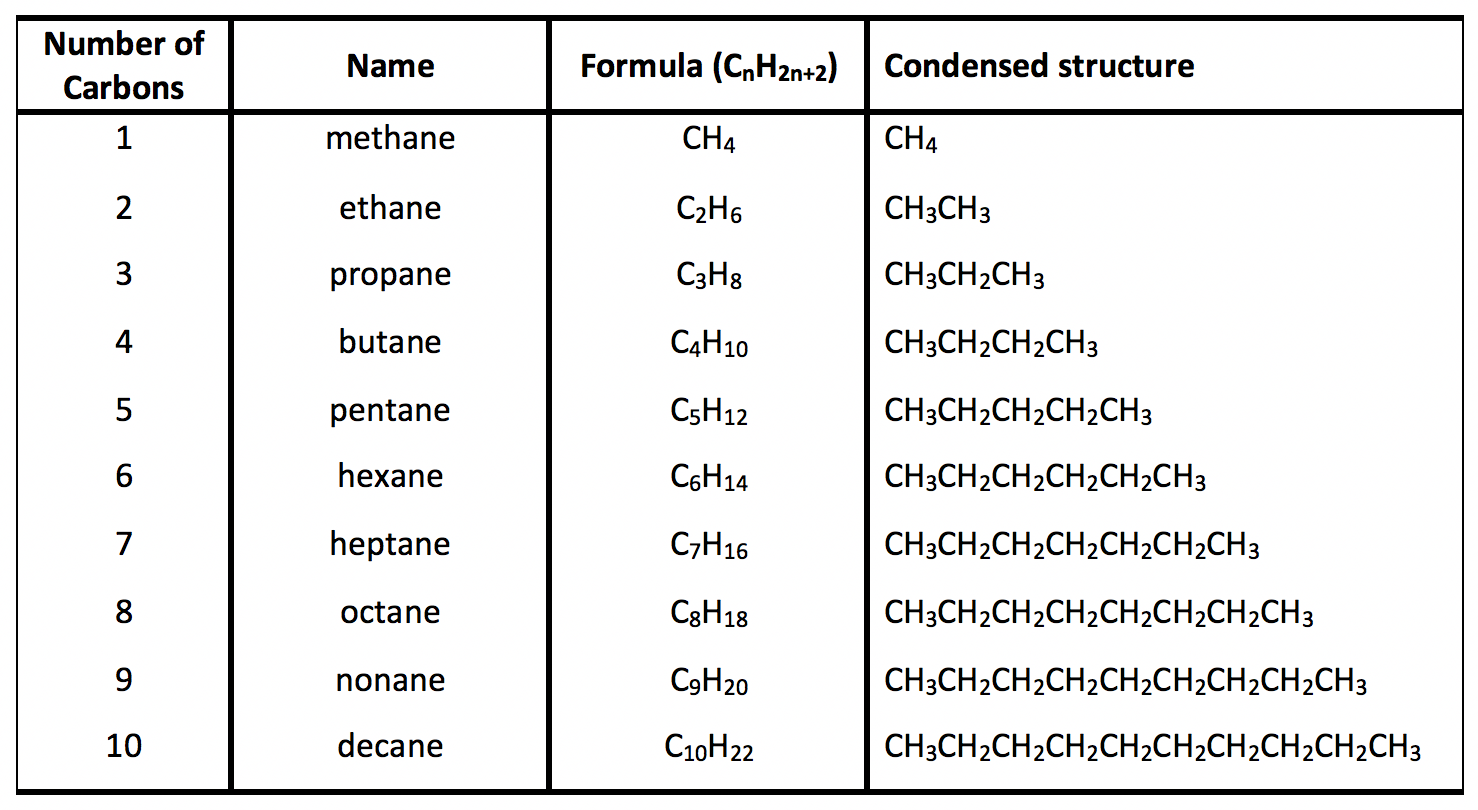
Alkane Alkene Alkyne Acidity . The acidity of the terminal alkynes is due to the high s characteristic of the sp. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. In other words, why are acetylide anions more stable than vinylic or. The hydrogen atom is bonded with a carbon atom in all three. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. Alkane, alkene and alkyne are hydrocarbons and there are different. When a terminal alkyne is treated with a. Why are terminal alkynes more acidic than alkenes or alkanes?
from kpu.pressbooks.pub
The hydrogen atom is bonded with a carbon atom in all three. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. Why are terminal alkynes more acidic than alkenes or alkanes? When a terminal alkyne is treated with a. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. In other words, why are acetylide anions more stable than vinylic or. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. The acidity of the terminal alkynes is due to the high s characteristic of the sp. Alkane, alkene and alkyne are hydrocarbons and there are different.
2.1 Structures of Alkenes Organic Chemistry
Alkane Alkene Alkyne Acidity The acidity of the terminal alkynes is due to the high s characteristic of the sp. When a terminal alkyne is treated with a. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. The acidity of the terminal alkynes is due to the high s characteristic of the sp. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. Alkane, alkene and alkyne are hydrocarbons and there are different. The hydrogen atom is bonded with a carbon atom in all three. Why are terminal alkynes more acidic than alkenes or alkanes? When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. In other words, why are acetylide anions more stable than vinylic or. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne.
From www.studypool.com
SOLUTION Alkane alkene alkyne reaction Studypool Alkane Alkene Alkyne Acidity The acidity of the terminal alkynes is due to the high s characteristic of the sp. In other words, why are acetylide anions more stable than vinylic or. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Why are terminal alkynes more acidic than alkenes or alkanes? The most. Alkane Alkene Alkyne Acidity.
From www.youtube.com
Alkanes, Alkenes, Alkynes (4/11) Organic Chemistry NCEA Level 2 Alkane Alkene Alkyne Acidity The acidity of the terminal alkynes is due to the high s characteristic of the sp. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. Alkane, alkene and alkyne are hydrocarbons and there are different. Why are terminal alkynes more acidic than alkenes or alkanes? When it comes to alkanes, alkenes, and alkynes, the. Alkane Alkene Alkyne Acidity.
From www.vedantu.com
Naming of Alkenes, Alkynes and Alkanes Important Concepts for JEE Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. Why are terminal alkynes more acidic than alkenes or alkanes? The hydrogen atom is bonded with a carbon atom in all three. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. In other words, why. Alkane Alkene Alkyne Acidity.
From www.doubtnut.com
Alkane and Alkene Alkane Alkene Alkyne Acidity The acidity of the terminal alkynes is due to the high s characteristic of the sp. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes >. Alkane Alkene Alkyne Acidity.
From selfstudypoint.in
Comparative Study of Alkanes, Alkenes, Alkynes Alkane Alkene Alkyne Acidity In other words, why are acetylide anions more stable than vinylic or. The hydrogen atom is bonded with a carbon atom in all three. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. Why are terminal alkynes more acidic than alkenes or alkanes? The acidity of the terminal alkynes is due to the high. Alkane Alkene Alkyne Acidity.
From quizlet.com
Naming Alkanes, Alkenes, and Alkynes Diagram Quizlet Alkane Alkene Alkyne Acidity When a terminal alkyne is treated with a. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. In other words, why are acetylide anions more stable than vinylic or. Why. Alkane Alkene Alkyne Acidity.
From www.slideshare.net
Alkane,alkene,alkyne Alkane Alkene Alkyne Acidity To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. Alkane, alkene and alkyne are hydrocarbons and there are different. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. The acidity of the. Alkane Alkene Alkyne Acidity.
From chemistnotes.com
Alkenes formula, structure, nomenclature, properties, and uses Alkane Alkene Alkyne Acidity Why are terminal alkynes more acidic than alkenes or alkanes? The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. When a terminal alkyne is treated with a. Alkane, alkene and alkyne are hydrocarbons and there are different. The acidity of the terminal alkynes is due to the high s characteristic of the sp. The. Alkane Alkene Alkyne Acidity.
From www.studypool.com
SOLUTION Alkane alkene alkyne complete note Studypool Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. The acidity of the terminal alkynes is due to the high s characteristic of the sp. Why are terminal alkynes more acidic than alkenes or alkanes? When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes >. Alkane Alkene Alkyne Acidity.
From www.chemistrysteps.com
Alkenes Structure and Stability Chemistry Steps Alkane Alkene Alkyne Acidity The acidity of the terminal alkynes is due to the high s characteristic of the sp. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. In other words, why are acetylide anions more stable than vinylic or. Alkane, alkene and alkyne are hydrocarbons and there are different. When a. Alkane Alkene Alkyne Acidity.
From www.youtube.com
Differences Between Alkane, Alkene, and Alkyne. YouTube Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. Why are terminal alkynes more acidic than alkenes or alkanes? When a terminal alkyne is treated with a. The hydrogen atom is bonded with a carbon atom in all three. In other words, why are acetylide anions more stable than vinylic or. Alkane, alkene and. Alkane Alkene Alkyne Acidity.
From www.youtube.com
Alkanes, Alkenes, and Alkynes General molecular formula Chemistry Alkane Alkene Alkyne Acidity In other words, why are acetylide anions more stable than vinylic or. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. When a terminal alkyne is treated with a. Alkane, alkene and alkyne are hydrocarbons and there are different. To introduce the hybridization effect, we will take a look. Alkane Alkene Alkyne Acidity.
From www.vrogue.co
Alkanes Alkenes And Alkynes General Molecular Formula vrogue.co Alkane Alkene Alkyne Acidity The hydrogen atom is bonded with a carbon atom in all three. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. The most striking difference between alkenes and alkynes is. Alkane Alkene Alkyne Acidity.
From www.shutterstock.com
Alkane Alkene Alkyne Functional Groups Organic Stock Illustration Alkane Alkene Alkyne Acidity To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. In other words, why are acetylide anions more stable than vinylic or. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. When it. Alkane Alkene Alkyne Acidity.
From www.vedantu.com
Naming of Alkenes, Alkynes and Alkanes Important Concepts for JEE Alkane Alkene Alkyne Acidity To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. The hydrogen atom is bonded with a carbon atom in all three. The acidity of the terminal alkynes is due to. Alkane Alkene Alkyne Acidity.
From simp-link.com
C9h18 alkane alkene or alkyne Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. The acidity of the terminal alkynes is due to the high s characteristic of the sp. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. To introduce. Alkane Alkene Alkyne Acidity.
From www.studypool.com
SOLUTION Wonder method of alkane alkene alkyne in organic chemistry Alkane Alkene Alkyne Acidity Alkane, alkene and alkyne are hydrocarbons and there are different. In other words, why are acetylide anions more stable than vinylic or. The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. When it comes to alkanes, alkenes, and alkynes, the acidity is. Alkane Alkene Alkyne Acidity.
From www.studypool.com
SOLUTION alkane alkene alkyne Studypool Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. The acidity of the terminal alkynes is due to the high s characteristic of the sp. When a terminal alkyne is treated with a. The. Alkane Alkene Alkyne Acidity.
From www.studypool.com
SOLUTION Alkane alkene alkyne Studypool Alkane Alkene Alkyne Acidity When a terminal alkyne is treated with a. The hydrogen atom is bonded with a carbon atom in all three. In other words, why are acetylide anions more stable than vinylic or. Alkane, alkene and alkyne are hydrocarbons and there are different. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. Why are terminal. Alkane Alkene Alkyne Acidity.
From www.studypool.com
SOLUTION Alkane ,alkene ,alkyne Studypool Alkane Alkene Alkyne Acidity When a terminal alkyne is treated with a. The acidity of the terminal alkynes is due to the high s characteristic of the sp. In other words, why are acetylide anions more stable than vinylic or. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Alkane, alkene and alkyne. Alkane Alkene Alkyne Acidity.
From www.youtube.com
Organic Chemistry Chemical Properties and AcidBase Reaction of Alkane Alkene Alkyne Acidity The acidity of the terminal alkynes is due to the high s characteristic of the sp. Why are terminal alkynes more acidic than alkenes or alkanes? When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively. Alkane Alkene Alkyne Acidity.
From slideplayer.com
Alkenes and Alkynes CHAPTER FOUR ppt download Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. In other words, why are acetylide anions more stable than vinylic or. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Why are terminal alkynes more acidic than alkenes or alkanes? The acidity of. Alkane Alkene Alkyne Acidity.
From www.youtube.com
Stability and Reactivity order of alkanes, alkenes and alkynes Alkane Alkene Alkyne Acidity The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. The hydrogen atom is bonded with a carbon atom in all three. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. In other. Alkane Alkene Alkyne Acidity.
From www.studypool.com
SOLUTION Alkane alkene alkyne Studypool Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. When a terminal alkyne is treated with a. The acidity of the terminal alkynes is due to the high s characteristic of the sp. The acidity. Alkane Alkene Alkyne Acidity.
From chemistnotes.com
Alkenes formula, structure, nomenclature, properties, and uses Alkane Alkene Alkyne Acidity Alkane, alkene and alkyne are hydrocarbons and there are different. When a terminal alkyne is treated with a. The acidity of the terminal alkynes is due to the high s characteristic of the sp. The hydrogen atom is bonded with a carbon atom in all three. To introduce the hybridization effect, we will take a look at the acidity difference. Alkane Alkene Alkyne Acidity.
From brainly.com
Question 1 Alkanes, Alkenes, and Alkynes Complete the table by Alkane Alkene Alkyne Acidity Alkane, alkene and alkyne are hydrocarbons and there are different. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. The acidity of the terminal alkynes is due to the high. Alkane Alkene Alkyne Acidity.
From kpu.pressbooks.pub
2.1 Structures of Alkenes Organic Chemistry Alkane Alkene Alkyne Acidity In other words, why are acetylide anions more stable than vinylic or. The hydrogen atom is bonded with a carbon atom in all three. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. The acidity of the terminal alkynes is due to the high s characteristic of the sp. Why. Alkane Alkene Alkyne Acidity.
From owlcation.com
The Chemistry of Alkenes Structure, Naming, Uses & Reactions Owlcation Alkane Alkene Alkyne Acidity The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. To introduce the hybridization effect, we will take a look at the acidity. Alkane Alkene Alkyne Acidity.
From www.studypool.com
SOLUTION Alkane alkene alkyne complete note Studypool Alkane Alkene Alkyne Acidity When a terminal alkyne is treated with a. Alkane, alkene and alkyne are hydrocarbons and there are different. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. Why are terminal alkynes more acidic than alkenes or alkanes? The acidity of the terminal alkynes is due to the high s. Alkane Alkene Alkyne Acidity.
From www.masterorganicchemistry.com
5 Key Factors That Influence Acidity In Organic Chemistry Alkane Alkene Alkyne Acidity To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. Why are terminal alkynes more acidic than alkenes or alkanes? In other words, why are acetylide anions more stable than vinylic or. When a terminal alkyne is treated with a. When it comes to alkanes, alkenes, and alkynes, the acidity is. Alkane Alkene Alkyne Acidity.
From www.meritnation.com
Draw the structure of first 5 members of alkanes,alkenes,alkynes Alkane Alkene Alkyne Acidity The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. The acidity of the terminal alkynes is due to the high s characteristic of the sp. When a terminal alkyne is treated with a. Why are terminal alkynes more acidic than alkenes or alkanes? The acidity of terminal alkynes is in fact pretty useful from. Alkane Alkene Alkyne Acidity.
From simp-link.com
C9h18 alkane alkene or alkyne Alkane Alkene Alkyne Acidity The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. The hydrogen atom is bonded with a carbon atom in all three. The. Alkane Alkene Alkyne Acidity.
From kindergartensheet.blogspot.com
Alkanes Alkenes Alkynes Worksheet Kindergarten Printable Sheet Alkane Alkene Alkyne Acidity The acidity of terminal alkynes is in fact pretty useful from a synthetic viewpoint, since you can deprotonate them with a strong base like $\ce{nanh2}$, forming a. The hydrogen atom is bonded with a carbon atom in all three. The acidity of the terminal alkynes is due to the high s characteristic of the sp. To introduce the hybridization effect,. Alkane Alkene Alkyne Acidity.
From www.youtube.com
C1d Alkanes and alkenes YouTube Alkane Alkene Alkyne Acidity When a terminal alkyne is treated with a. The most striking difference between alkenes and alkynes is that terminal alkynes are relatively acidic. To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. In other words, why are acetylide anions more stable than vinylic or. The acidity of the terminal alkynes. Alkane Alkene Alkyne Acidity.
From www.masterorganicchemistry.com
5 Key Basicity Trends of Amines Master Organic Chemistry Alkane Alkene Alkyne Acidity To introduce the hybridization effect, we will take a look at the acidity difference between alkane, alkene and alkyne. When it comes to alkanes, alkenes, and alkynes, the acidity is in the order of alkynes > alkenes > alkanes. When a terminal alkyne is treated with a. The acidity of terminal alkynes is in fact pretty useful from a synthetic. Alkane Alkene Alkyne Acidity.