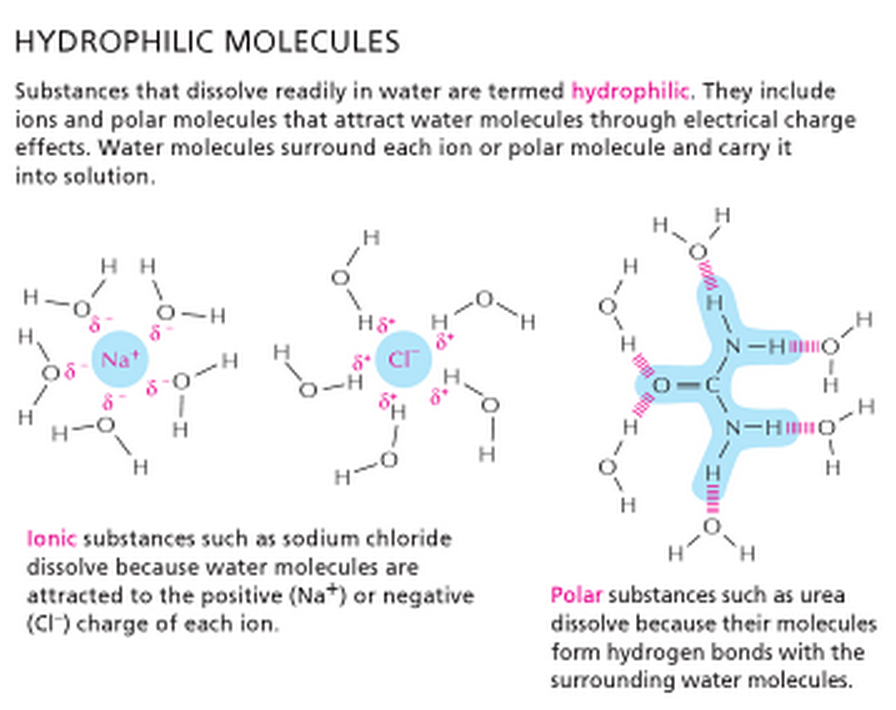
Hydrophilic Molecules Quizlet . When a substance readily forms hydrogen bonds with. These molecules must have a charge (positive or negative) in order to interact with water, which is. They dissolve readily in water. The plasma membrane consists of hydrophobic and hydrophillic characteristics. Hydrophilic molecules are molecules that can dissolve in water. Towards the outsides, they are hydrophillic, so. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. A) are uncharged, nonionic substances that repel water. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. B) are nonionic molecules that are attracted to. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and.
from joannelovesscience.com
A) are uncharged, nonionic substances that repel water. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. They dissolve readily in water. When a substance readily forms hydrogen bonds with. Hydrophilic molecules are molecules that can dissolve in water. B) are nonionic molecules that are attracted to. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. Towards the outsides, they are hydrophillic, so. The plasma membrane consists of hydrophobic and hydrophillic characteristics. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils.
Chemistry of Mascara Joanne Loves Science
Hydrophilic Molecules Quizlet Hydrophilic molecules are molecules that can dissolve in water. A) are uncharged, nonionic substances that repel water. Hydrophilic molecules are molecules that can dissolve in water. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. Towards the outsides, they are hydrophillic, so. The plasma membrane consists of hydrophobic and hydrophillic characteristics. These molecules must have a charge (positive or negative) in order to interact with water, which is. B) are nonionic molecules that are attracted to. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. When a substance readily forms hydrogen bonds with. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. They dissolve readily in water.
From quizlet.com
Draw a sketch to show how the hydrophilic and hydrophobic pa Quizlet Hydrophilic Molecules Quizlet B) are nonionic molecules that are attracted to. Towards the outsides, they are hydrophillic, so. A) are uncharged, nonionic substances that repel water. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. These molecules must have a charge (positive or negative) in order to interact with water, which is. Water also attracts. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Classify each molecule as hydrophilic, hydrophobic, Hydrophilic Molecules Quizlet When a substance readily forms hydrogen bonds with. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. They dissolve readily in water. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Towards the outsides, they are hydrophillic, so. A) are uncharged, nonionic substances that repel water. The plasma membrane. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Question Hydrophilic Molecules Quizlet Towards the outsides, they are hydrophillic, so. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. These molecules must have a charge (positive or negative) in order to interact with water, which is. Hydrophilic molecules are molecules that can dissolve in water. Water also attracts other polar molecules (such as sugars),. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Which molecule has more hydrophilic properties? A) Hydrophilic Molecules Quizlet B) are nonionic molecules that are attracted to. They dissolve readily in water. When a substance readily forms hydrogen bonds with. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. Molecules like sugars (carbohydrates),. Hydrophilic Molecules Quizlet.
From entreasmemorias.blogspot.com
70 Awesome Is Water Hydrophobic Or Hydrophilic Quizlet insectza Hydrophilic Molecules Quizlet Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. These molecules must have a charge (positive or negative) in order to interact with water, which is. Hydrophilic molecules are molecules that can dissolve in water. A) are uncharged, nonionic substances that repel water. Hydrophobic molecules. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Highlight the atoms or groups that are in hydrophilic Hydrophilic Molecules Quizlet Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Hydrophilic molecules are molecules that can dissolve in water. A) are uncharged, nonionic substances that repel water. They dissolve readily in water. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as. Hydrophilic Molecules Quizlet.
From www.numerade.com
SOLVED Hydrophilic or Hydrophobic Choose which substances are Hydrophilic Molecules Quizlet Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. When a substance readily forms hydrogen bonds with. Towards the outsides, they are hydrophillic, so. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. These molecules must have. Hydrophilic Molecules Quizlet.
From www.numerade.com
SOLVED Question 5 What kind of molecule is shown below? HaNt Cc CHz Hydrophilic Molecules Quizlet Hydrophilic molecules are molecules that can dissolve in water. A) are uncharged, nonionic substances that repel water. The plasma membrane consists of hydrophobic and hydrophillic characteristics. B) are nonionic molecules that are attracted to. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Study with quizlet and memorize flashcards containing terms like. Hydrophilic Molecules Quizlet.
From www.numerade.com
SOLVEDDescribe how hydrophilic and hydrophobic colloids are stabilized Hydrophilic Molecules Quizlet B) are nonionic molecules that are attracted to. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. They dissolve readily in water. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Hydrophilic molecules are molecules that can dissolve in water. A) are uncharged, nonionic substances that repel water. These. Hydrophilic Molecules Quizlet.
From animalia-life.club
Hydrophilic Examples Hydrophilic Molecules Quizlet Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. The plasma membrane consists of hydrophobic and hydrophillic characteristics. Towards the outsides, they are hydrophillic, so. Hydrophilic molecules are molecules that can dissolve in water. When a substance readily forms hydrogen bonds with. A) are uncharged, nonionic substances that repel water. These molecules must have a charge (positive. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Classify each molecule as hydrophilic, hydrophobic, Hydrophilic Molecules Quizlet B) are nonionic molecules that are attracted to. These molecules must have a charge (positive or negative) in order to interact with water, which is. When a substance readily forms hydrogen bonds with. A) are uncharged, nonionic substances that repel water. Hydrophilic molecules are molecules that can dissolve in water. Study with quizlet and memorize flashcards containing terms like hydrophobic,. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Is the molecule shown hydrophilic, hydrophobic, or Hydrophilic Molecules Quizlet These molecules must have a charge (positive or negative) in order to interact with water, which is. Towards the outsides, they are hydrophillic, so. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. When a substance readily forms hydrogen bonds with. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily. Hydrophilic Molecules Quizlet.
From www.numerade.com
SOLVED Text Label each of these biomolecules as charged polar Hydrophilic Molecules Quizlet The plasma membrane consists of hydrophobic and hydrophillic characteristics. These molecules must have a charge (positive or negative) in order to interact with water, which is. B) are nonionic molecules that are attracted to. A) are uncharged, nonionic substances that repel water. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and.. Hydrophilic Molecules Quizlet.
From courses.lumenlearning.com
Components and Structure OpenStax Biology 2e Hydrophilic Molecules Quizlet Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. The plasma membrane consists of hydrophobic and hydrophillic characteristics. Hydrophilic molecules are molecules that can dissolve in water. Towards the outsides, they are hydrophillic, so. Water also attracts other polar. Hydrophilic Molecules Quizlet.
From www.biologyonline.com
Hydrophilic Definition and Examples Biology Online Dictionary Hydrophilic Molecules Quizlet B) are nonionic molecules that are attracted to. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. These molecules must have a charge (positive or negative) in order to interact with water, which is. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Towards the outsides, they are hydrophillic,. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Classify each molecule as hydrophilic, hydrophobic, Hydrophilic Molecules Quizlet B) are nonionic molecules that are attracted to. When a substance readily forms hydrogen bonds with. A) are uncharged, nonionic substances that repel water. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. These molecules must have a charge (positive or negative) in order to interact with water, which is. Water. Hydrophilic Molecules Quizlet.
From www.pinterest.co.uk
Hydrophobic vs hydrophilic surface effect on water drop outline diagram Hydrophilic Molecules Quizlet The plasma membrane consists of hydrophobic and hydrophillic characteristics. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Towards the outsides, they are hydrophillic, so. B) are nonionic. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Is the following molecule hydrophilic, hydrophobic, Hydrophilic Molecules Quizlet The plasma membrane consists of hydrophobic and hydrophillic characteristics. Towards the outsides, they are hydrophillic, so. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. A) are uncharged, nonionic substances that repel water. Hydrophilic molecules are molecules. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Is the molecule, shown below hydrophilic, Hydrophilic Molecules Quizlet Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. Towards the outsides, they are hydrophillic, so. These molecules must have a charge (positive or negative) in order to interact with water, which is. A) are uncharged, nonionic substances that repel water. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. The plasma membrane consists. Hydrophilic Molecules Quizlet.
From animalia-life.club
Hydrophilic Examples Hydrophilic Molecules Quizlet Towards the outsides, they are hydrophillic, so. The plasma membrane consists of hydrophobic and hydrophillic characteristics. When a substance readily forms hydrogen bonds with. Hydrophilic molecules are molecules that can dissolve in water. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats. Hydrophilic Molecules Quizlet.
From microbenotes.com
Hydrophilic Molecule Definition, Examples, Applications Hydrophilic Molecules Quizlet These molecules must have a charge (positive or negative) in order to interact with water, which is. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. They dissolve readily in water. The plasma membrane consists of hydrophobic and hydrophillic characteristics. B) are nonionic molecules that are attracted to. Study with quizlet and memorize flashcards containing terms like. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Classify each molecule as hydrophilic, hydrophobic or Hydrophilic Molecules Quizlet Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. When a substance readily forms hydrogen bonds with. These molecules must have a charge (positive or negative) in order to interact with water, which is. Hydrophilic molecules are molecules that can. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Classify the three molecules as hydrophilic, Hydrophilic Molecules Quizlet A) are uncharged, nonionic substances that repel water. These molecules must have a charge (positive or negative) in order to interact with water, which is. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. Towards the outsides, they are. Hydrophilic Molecules Quizlet.
From quizlet.com
Which of the 20 amino acids have a hydrophilic side chain? Quizlet Hydrophilic Molecules Quizlet Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. A) are uncharged, nonionic substances that repel water. The plasma membrane consists of hydrophobic and hydrophillic characteristics. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with. Hydrophilic molecules are molecules that. Hydrophilic Molecules Quizlet.
From joannelovesscience.com
Chemistry of Mascara Joanne Loves Science Hydrophilic Molecules Quizlet Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Hydrophilic molecules are molecules that can dissolve in water. The plasma membrane consists of hydrophobic and hydrophillic characteristics. Towards the outsides, they are hydrophillic, so. They dissolve readily in water. B) are nonionic molecules that are attracted to. A) are uncharged, nonionic substances. Hydrophilic Molecules Quizlet.
From www.chegg.com
Identify the hydrophilic portion of the molecule by Hydrophilic Molecules Quizlet The plasma membrane consists of hydrophobic and hydrophillic characteristics. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Hydrophilic molecules are molecules that can dissolve in water. They dissolve readily in water. A) are uncharged, nonionic substances that repel water. Towards the outsides, they are hydrophillic, so. Hydrophobic molecules are nonpolar molecules that do not interact well. Hydrophilic Molecules Quizlet.
From quizlet.com
Lecture 1 Biological Molecules and Functional Groups Diagram Quizlet Hydrophilic Molecules Quizlet Hydrophilic molecules are molecules that can dissolve in water. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with. A) are uncharged, nonionic substances that repel water. They dissolve readily in water. B). Hydrophilic Molecules Quizlet.
From quizlet.com
Hydrophobic and hydrophilic signal steps Diagram Quizlet Hydrophilic Molecules Quizlet When a substance readily forms hydrogen bonds with. They dissolve readily in water. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. These molecules must have a charge (positive or negative) in order to interact with water, which is. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. The plasma membrane consists of hydrophobic. Hydrophilic Molecules Quizlet.
From quizlet.com
human bio test 1 Flashcards Quizlet Hydrophilic Molecules Quizlet These molecules must have a charge (positive or negative) in order to interact with water, which is. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Hydrophilic molecules are molecules that can dissolve in water. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Study with quizlet and memorize. Hydrophilic Molecules Quizlet.
From variosmodelo.blogspot.com
Describe The Fluid Mosaic Model Of Membrane Structure Quizlet Vários Hydrophilic Molecules Quizlet When a substance readily forms hydrogen bonds with. These molecules must have a charge (positive or negative) in order to interact with water, which is. The plasma membrane consists of hydrophobic and hydrophillic characteristics. B) are nonionic molecules that are attracted to. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and.. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Highlight the atoms or groups that are in hydrophilic Hydrophilic Molecules Quizlet Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. Hydrophilic molecules are molecules that can dissolve in water. Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. When a substance readily. Hydrophilic Molecules Quizlet.
From www.chegg.com
Solved Classify each molecule as hydrophilic, hydrophobic, Hydrophilic Molecules Quizlet Hydrophobic molecules are nonpolar molecules that do not interact well with water, such as fats and oils. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. These molecules must have a charge (positive or negative) in order to interact with water, which is. B) are nonionic molecules that are attracted to. They dissolve readily in water. Study. Hydrophilic Molecules Quizlet.
From quizlet.com
Hydrophilic amino acids Diagram Quizlet Hydrophilic Molecules Quizlet They dissolve readily in water. When a substance readily forms hydrogen bonds with. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. The plasma membrane consists of hydrophobic and hydrophillic characteristics. These molecules must have a charge (positive or negative) in order to interact with water, which is. Hydrophobic molecules are nonpolar molecules that do not interact. Hydrophilic Molecules Quizlet.
From quizlet.com
Hydrophilic Hormone Action Diagram Quizlet Hydrophilic Molecules Quizlet B) are nonionic molecules that are attracted to. They dissolve readily in water. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic, how does polarity relate to hydrophilic and. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. A) are uncharged, nonionic substances that repel water. The plasma membrane consists of hydrophobic and hydrophillic characteristics.. Hydrophilic Molecules Quizlet.
From quizlet.com
Hydrophilic Hormones Diagram Quizlet Hydrophilic Molecules Quizlet A) are uncharged, nonionic substances that repel water. Hydrophilic molecules are molecules that can dissolve in water. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. B) are nonionic molecules that are attracted to. Molecules like sugars (carbohydrates), alcohols, and most amino acids are primarily hydrophilic. Study with quizlet and memorize flashcards containing terms like hydrophobic, hydrophilic,. Hydrophilic Molecules Quizlet.