Lead + Hcl Equation . The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: The balanced equation will be calculated along with the. Balance any equation or reaction using this chemical equation balancer! Enter an equation of an ionic chemical equation and press the balance button. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Net ionic equations show only the ions and other substances that change in a chemical reaction. Complete ionic equations show dissolved ionic solids as separated ions. Find out what type of reaction occured. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:
from www.youtube.com
Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The balanced equation will be calculated along with the. The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: Net ionic equations show only the ions and other substances that change in a chemical reaction. Enter an equation of an ionic chemical equation and press the balance button. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Complete ionic equations show dissolved ionic solids as separated ions. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:
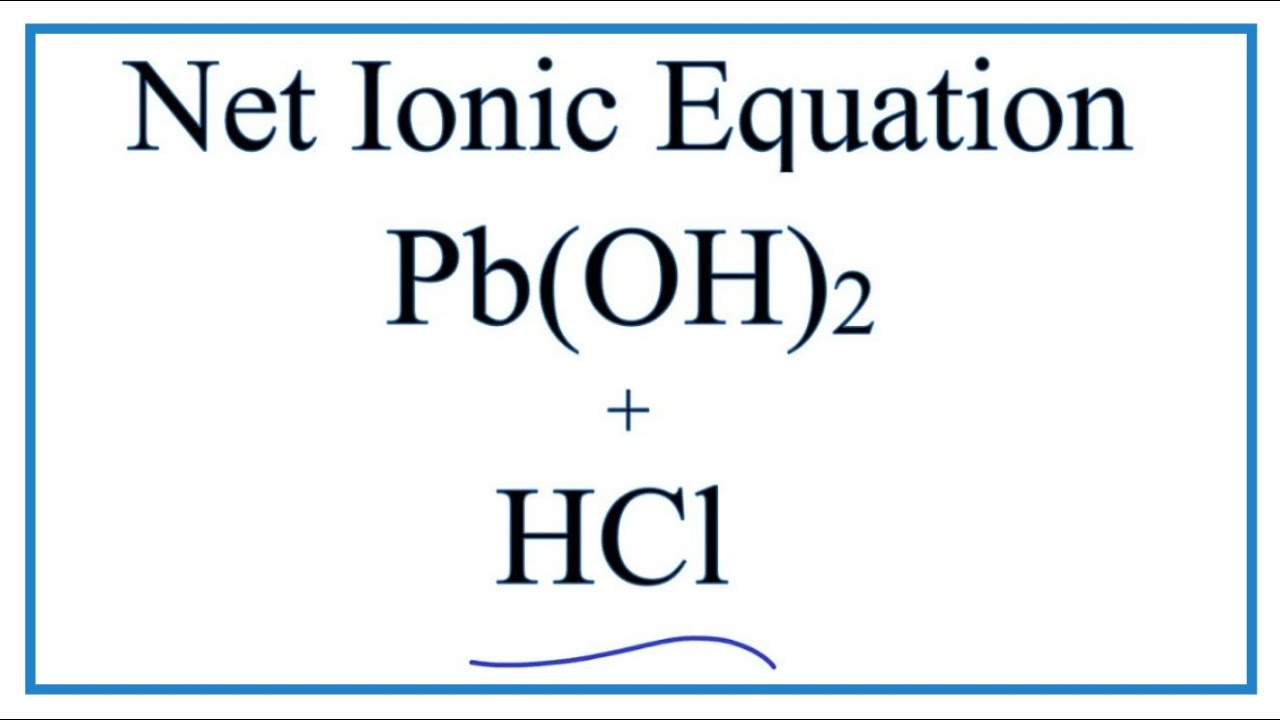
How to Write the Net Ionic Equation for Pb(OH)2 + HCl = H2O + PbCl2
Lead + Hcl Equation Find out what type of reaction occured. The balanced equation will be calculated along with the. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Net ionic equations show only the ions and other substances that change in a chemical reaction. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Complete ionic equations show dissolved ionic solids as separated ions. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is:
From www.youtube.com
Lead and HCl YouTube Lead + Hcl Equation Balance any equation or reaction using this chemical equation balancer! This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Find out what type of reaction occured. Complete ionic equations show dissolved ionic solids as separated ions. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric. Lead + Hcl Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Lead + Hcl Equation Complete ionic equations show dissolved ionic solids as separated ions. Net ionic equations show only the ions and other substances that change in a chemical reaction. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Find out what type of reaction occured. Balance any equation or reaction. Lead + Hcl Equation.
From www.coursehero.com
[Solved] Solve the following thermochemical reactions 2 HCl + Pb Lead + Hcl Equation Net ionic equations show only the ions and other substances that change in a chemical reaction. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Balance any equation or reaction using this chemical equation balancer! The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: This. Lead + Hcl Equation.
From www.pinterest.com
How to Balance Pb(OH)2 + HCl = H2O + PbCl2 Balancing equations, Math Lead + Hcl Equation Find out what type of reaction occured. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press. Lead + Hcl Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Pb(OH)2 + HCl = H2O + PbCl2 Lead + Hcl Equation The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: Find out what type of reaction occured. Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the formation of. Lead + Hcl Equation.
From www.youtube.com
How to Balance Pb(NO3)2 + HCl = PbCl2 + HNO3 YouTube Lead + Hcl Equation Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Complete ionic equations show dissolved ionic solids as separated ions. Enter an equation of an ionic chemical equation and press the balance button. Net ionic equations show only the ions and other substances that change in a chemical reaction. Balance any. Lead + Hcl Equation.
From www.coursehero.com
[Solved] For C, Does Cu(NO3)2+HCl have a net ionic equation even though Lead + Hcl Equation This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Find out what type of reaction occured. The balanced equation will be calculated along with the. Net ionic equations show only the ions. Lead + Hcl Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Lead + Hcl Equation This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using. Lead + Hcl Equation.
From mammothmemory.net
Leads reaction with hydrochloric acid is very slow indeed Lead + Hcl Equation Complete ionic equations show dissolved ionic solids as separated ions. Net ionic equations show only the ions and other substances that change in a chemical reaction. The balanced equation will be calculated along with the. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Lead(ii) ion reacts with aqueous. Lead + Hcl Equation.
From www.youtube.com
Pb(OH)2+HCl=H2O+PbCl2 Balanced EquationLead hydroxide+Hydrochloric Lead + Hcl Equation Find out what type of reaction occured. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The balanced equation will be calculated along with the. Net ionic equations show only the ions and other substances that change in a chemical reaction. Enter an equation of an ionic chemical equation and press. Lead + Hcl Equation.
From www.youtube.com
How to balance Pb(OH)2+HCl=PbCl2+H2OChemical equation Pb(OH)2+HCl Lead + Hcl Equation Net ionic equations show only the ions and other substances that change in a chemical reaction. Find out what type of reaction occured. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Complete ionic equations show dissolved ionic solids as separated ions. The balanced equation for the reaction of sulfur. Lead + Hcl Equation.
From studylib.net
Net Ionic Equations Lead + Hcl Equation The balanced equation will be calculated along with the. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Enter an equation of an ionic chemical equation and press the balance button. Net ionic equations. Lead + Hcl Equation.
From www.youtube.com
how to balance Pb (NO3)2+HCl=PbCl2+HNO3Chemical equation Pb (NO3)2+HCl Lead + Hcl Equation Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Complete ionic equations show dissolved ionic solids as separated ions. Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). Lead + Hcl Equation.
From www.youtube.com
How to Write the Formula for Lead (IV) oxide YouTube Lead + Hcl Equation Find out what type of reaction occured. Net ionic equations show only the ions and other substances that change in a chemical reaction. The balanced equation will be calculated along with the. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Balance any equation or reaction using this chemical equation balancer!. Lead + Hcl Equation.
From www.numerade.com
SOLVED Balance the Chemical Equation Pb(OH)2 + HCl —> H2O + PbCl2 Lead + Hcl Equation Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer! Complete ionic equations show dissolved ionic solids as separated ions. This page looks at the formation of. Lead + Hcl Equation.
From www.slideserve.com
PPT Net Ionic Equations PowerPoint Presentation, free download ID Lead + Hcl Equation Balance any equation or reaction using this chemical equation balancer! This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in. Lead + Hcl Equation.
From www.youtube.com
Electrolysis of Lead ii Bromide and Concentrated hydrochloric acid Lead + Hcl Equation Complete ionic equations show dissolved ionic solids as separated ions. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Balance any equation or reaction using this chemical equation balancer! Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with. Lead + Hcl Equation.
From www.youtube.com
Lead(Pb)+Dilute hydrochloric acid (HCl) The Balanced chemical Equation Lead + Hcl Equation Complete ionic equations show dissolved ionic solids as separated ions. Net ionic equations show only the ions and other substances that change in a chemical reaction. Enter an equation of an ionic chemical equation and press the balance button. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The balanced equation. Lead + Hcl Equation.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Lead + Hcl Equation Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. The balanced equation will be calculated along with the. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Net ionic equations show only the ions and other substances that change in a chemical. Lead + Hcl Equation.
From www.chegg.com
Solved Date Name 1. Lead will react with hydrochloric acid Lead + Hcl Equation The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: Complete ionic equations show dissolved ionic solids as separated ions. Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Lead(ii) ion reacts. Lead + Hcl Equation.
From www.youtube.com
Type of Reaction for Pb(OH)2 + HCl = PbCl2 + H2O YouTube Lead + Hcl Equation This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Find out what type of reaction occured. The balanced equation will be calculated along with the. Net ionic equations show only the ions and other substances that change in a chemical reaction. Lead(ii) ion reacts with aqueous ammonia to precipitate a white. Lead + Hcl Equation.
From www.showme.com
ShowMe net ionic equation Lead + Hcl Equation The balanced equation will be calculated along with the. Complete ionic equations show dissolved ionic solids as separated ions. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Net ionic equations show only the. Lead + Hcl Equation.
From www.chegg.com
Solved Solid lead(II) sulfide reacts with aqueous Lead + Hcl Equation Complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead(ii) chloride, a white precipitate, is formed by adding a. Lead + Hcl Equation.
From www.toppr.com
Write a balanced equation for the followingRed lead is warmed with Lead + Hcl Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Find out what type of reaction occured. Complete ionic equations show dissolved ionic. Lead + Hcl Equation.
From www.tessshebaylo.com
Write An Equation Showing The Ionization Of Hydrochloric Acid In Water Lead + Hcl Equation Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Net ionic equations show only the ions and other substances that change in a chemical reaction. The balanced equation will be calculated along with the. Complete ionic equations show dissolved ionic solids as separated ions. Lead(ii) chloride, a white precipitate, is. Lead + Hcl Equation.
From www.numerade.com
SOLVED Lead (II) oxide reacts with hydrochloric acid in a double Lead + Hcl Equation Balance any equation or reaction using this chemical equation balancer! Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Complete ionic equations show dissolved ionic solids as. Lead + Hcl Equation.
From www.youtube.com
How to Write the Net Ionic Equation for HClO3 + NaOH = NaClO3 + H2O Lead + Hcl Equation Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Find out what type of reaction occured. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be. Lead + Hcl Equation.
From socratic.org
What is the molecular and net ionic equation for the reaction between Lead + Hcl Equation Complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be calculated along with the. Net ionic equations show only the ions and other substances that change in a chemical reaction. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for the reaction of sulfur (s) and hydrochloric acid. Lead + Hcl Equation.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Lead + Hcl Equation Net ionic equations show only the ions and other substances that change in a chemical reaction. Find out what type of reaction occured. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: The balanced equation will be calculated along with the. Lead(ii). Lead + Hcl Equation.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead + Hcl Equation Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Find out what type of reaction occured. Net ionic equations show only the ions and other substances that change in a chemical reaction. Complete ionic equations show dissolved ionic solids as separated ions. Enter an equation of an ionic chemical equation. Lead + Hcl Equation.
From www.youtube.com
Ag + HCl (Silver + Hydrochloric acid) Equation YouTube Lead + Hcl Equation Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Find out what type of reaction occured. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Net ionic equations show only the ions and other substances that. Lead + Hcl Equation.
From www.solutionspile.com
[Solved] Lead (IV) oxide dissolves in concentrated hydroc Lead + Hcl Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. Complete ionic equations show dissolved ionic solids as separated ions. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The balanced equation for the reaction of sulfur. Lead + Hcl Equation.
From brainly.in
Sodium chloride solution is added to lead nitrate solution. Also write Lead + Hcl Equation Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Balance any equation or reaction using this chemical equation balancer! Net ionic equations show only the ions and other substances that. Lead + Hcl Equation.
From slideplayer.com
Metals and Metal Compounds ppt download Lead + Hcl Equation Complete ionic equations show dissolved ionic solids as separated ions. The balanced equation will be calculated along with the. Net ionic equations show only the ions and other substances that change in a chemical reaction. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The balanced equation for the. Lead + Hcl Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Lead + Hcl Equation The balanced equation will be calculated along with the. The balanced equation for the reaction of sulfur (s) and hydrochloric acid (hcl) is: Net ionic equations show only the ions and other substances that change in a chemical reaction. Find out what type of reaction occured. This page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii). Lead + Hcl Equation.