What Is A Mole Used To Measure In Chemistry . Learn how the mole is defined,. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Learn how to calculate the mole, mass, and molecular weight of a. A mole is a unit of quantity that describes the number of particles in a system. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to calculate moles, molar mass, and. Mole calculations using balanced equations mass to moles calculations. The mass contained in one. Chemical amounts are measured in moles. Learn how to use the mole to calculate mass, number of molecules, and molar mass. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles.
from studylib.net
Learn how to use the mole to calculate mass, number of molecules, and molar mass. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Chemical amounts are measured in moles. Learn how the mole is defined,. Mole calculations using balanced equations mass to moles calculations. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. Learn how to calculate the mole, mass, and molecular weight of a. The mass contained in one. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Learn how to calculate moles, molar mass, and.
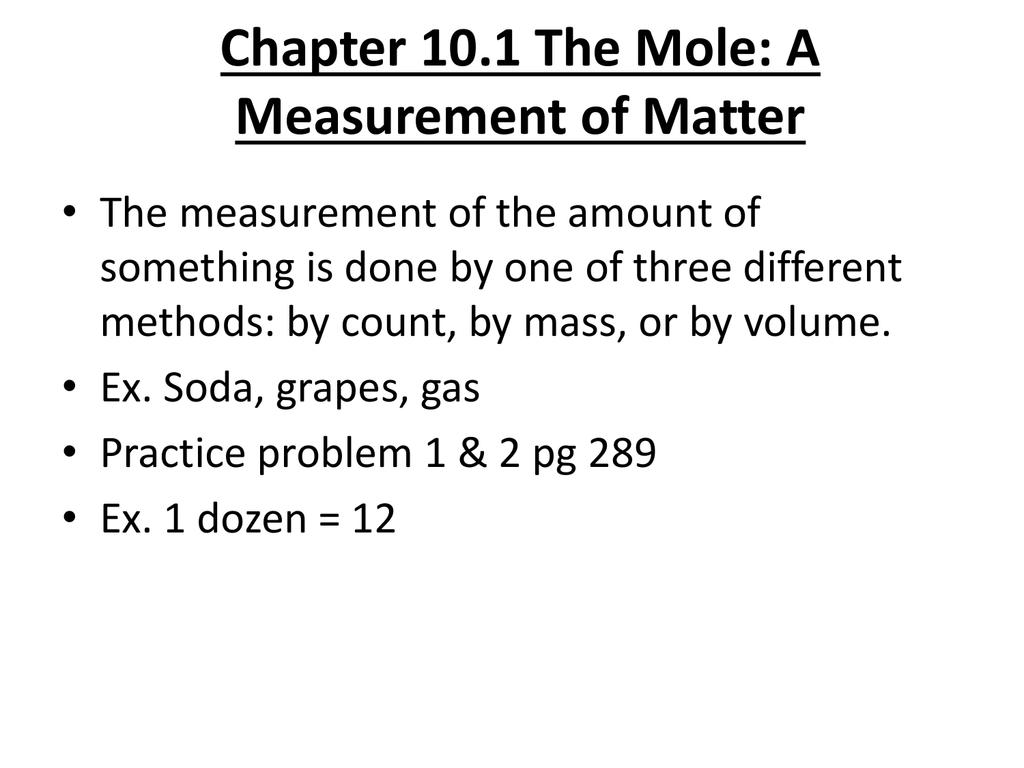
Chapter 10.1 The Mole A Measurement of Matter
What Is A Mole Used To Measure In Chemistry Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. Mole calculations using balanced equations mass to moles calculations. Learn how to calculate moles, molar mass, and. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. A mole is a unit of quantity that describes the number of particles in a system. Learn how to use the mole to calculate mass, number of molecules, and molar mass. Learn how to calculate the mole, mass, and molecular weight of a. Chemical amounts are measured in moles. The mass contained in one. Learn how the mole is defined,.
From periodictable.me
Introduction to the Mole in Chemistry A Brief Explanation What Is A Mole Used To Measure In Chemistry Learn how to calculate moles, molar mass, and. Chemical amounts are measured in moles. Learn how to calculate the mole, mass, and molecular weight of a. The mass contained in one. A mole is a unit of quantity that describes the number of particles in a system. A mole is a unit of measurement for counting atoms, molecules, or ions. What Is A Mole Used To Measure In Chemistry.
From slidetodoc.com
The Mole A Measurement of Matter Forensic ChemistryMrs What Is A Mole Used To Measure In Chemistry Learn how the mole is defined,. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. A mole is a unit of quantity that describes the number of particles in a system. Chemical amounts are measured in moles. Learn how to calculate moles, molar mass, and. Learn how to use the mole to calculate. What Is A Mole Used To Measure In Chemistry.
From www.thoughtco.com
What Is a Mole in Chemistry? What Is A Mole Used To Measure In Chemistry Learn how the mole is defined,. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Mole calculations using balanced equations mass to moles calculations. The mass contained in one. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. Learn how to use the mole concept. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT Calculations in Chemistry PowerPoint Presentation, free download What Is A Mole Used To Measure In Chemistry A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. A mole is a unit of quantity that describes the number of particles in a system. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. Learn how to use the mole to calculate mass,. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT Moles a counting unit in chemistry What is a mole? Why do we What Is A Mole Used To Measure In Chemistry Chemical amounts are measured in moles. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. The mass contained in one. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to calculate the mole, mass, and molecular weight of a. Learn how to use. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT General Chemistry I Virginia State University PowerPoint What Is A Mole Used To Measure In Chemistry Mole calculations using balanced equations mass to moles calculations. A mole is a unit of quantity that describes the number of particles in a system. Learn how the mole is defined,. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to use the mole concept and molar mass to calculate the amount. What Is A Mole Used To Measure In Chemistry.
From biobeat.nigms.nih.gov
In Other Words The Measure of a Mole Biomedical Beat Blog National What Is A Mole Used To Measure In Chemistry Learn how to use the mole to calculate mass, number of molecules, and molar mass. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. Learn how the mole is defined,. A mole is a. What Is A Mole Used To Measure In Chemistry.
From studylib.net
Chapter 10.1 The Mole A Measurement of Matter What Is A Mole Used To Measure In Chemistry A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. The mass contained in one. Chemical amounts are measured in moles. Learn how the mole is defined,. Mole calculations using balanced equations mass to moles calculations. A mole is a unit of quantity that describes the number of particles in a system. Learn how. What Is A Mole Used To Measure In Chemistry.
From www.templateroller.com
Chemistry Cheat Sheet Mole Conversion Chart Download Printable PDF What Is A Mole Used To Measure In Chemistry Learn how to use the mole to calculate mass, number of molecules, and molar mass. Learn how to calculate moles, molar mass, and. The mass contained in one. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. A mole is a unit of quantity that describes the number of. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT MOLE RATIOS IN CHEMICAL EQUATIONS PowerPoint Presentation, free What Is A Mole Used To Measure In Chemistry A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. Learn how the mole is defined,. The mass contained in one. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. A mole is a unit of quantity that describes the number of particles in a system.. What Is A Mole Used To Measure In Chemistry.
From quizlet.com
Chem 08 Reactions & Moles Diagram Quizlet What Is A Mole Used To Measure In Chemistry Learn how to calculate the mole, mass, and molecular weight of a. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. The mass contained in one. Learn how the mole is defined,. A mole is a unit of quantity that describes the number of particles in a system. A. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT Unit 4 The Mole PowerPoint Presentation, free download ID5601061 What Is A Mole Used To Measure In Chemistry Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to calculate the mole, mass, and molecular. What Is A Mole Used To Measure In Chemistry.
From www.pinterest.com
Mole Conversion Worksheet and Activity ⋆ Teaching What Is A Mole Used To Measure In Chemistry Learn how to calculate the mole, mass, and molecular weight of a. Chemical amounts are measured in moles. Learn how the mole is defined,. Learn how to calculate moles, molar mass, and. A mole is a unit of quantity that describes the number of particles in a system. A mole is a unit of measurement for counting atoms, molecules, or. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT The Mole A Measurement of Matter PowerPoint Presentation, free What Is A Mole Used To Measure In Chemistry A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. Mole calculations using balanced equations mass to moles calculations. Chemical amounts are measured in moles. Learn how to calculate the mole, mass, and molecular weight of a. The mass contained in one. Learn how to use the mole concept and molar mass. What Is A Mole Used To Measure In Chemistry.
From slidetodoc.com
THE MOLE Measuring Matter What is a mole What Is A Mole Used To Measure In Chemistry Learn how to use the mole to calculate mass, number of molecules, and molar mass. Mole calculations using balanced equations mass to moles calculations. Learn how the mole is defined,. Learn how to calculate moles, molar mass, and. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. A mole is a unit of. What Is A Mole Used To Measure In Chemistry.
From chemistryqa.com
What is a Mole in Chemistry? An InDepth Explanation What Is A Mole Used To Measure In Chemistry Learn how to use the mole to calculate mass, number of molecules, and molar mass. The mass contained in one. Chemical amounts are measured in moles. Learn how the mole is defined,. Learn how to calculate the mole, mass, and molecular weight of a. Mole calculations using balanced equations mass to moles calculations. A mole is a unit of measurement. What Is A Mole Used To Measure In Chemistry.
From www.thesciencehive.co.uk
Chemical formulae, equations and calculations GCSE — the science sauce What Is A Mole Used To Measure In Chemistry The mass contained in one. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Chemical amounts are measured in moles. Mole calculations using balanced equations mass to moles calculations. A mole is a unit of quantity that describes the number of particles in a system. Learn how the mole is defined,. Learn how. What Is A Mole Used To Measure In Chemistry.
From www.pinterest.com
A mole conversion is when the mole is able the measure both mass and What Is A Mole Used To Measure In Chemistry The mass contained in one. Learn how to calculate the mole, mass, and molecular weight of a. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. A mole is a unit of quantity that describes the number of particles in a system. A mole is a unit of measurement. What Is A Mole Used To Measure In Chemistry.
From thechemguys.blogspot.com
Chemestry 11 Lessons Mole Conversions What Is A Mole Used To Measure In Chemistry A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Learn how to calculate the mole, mass, and molecular weight of a. Mole calculations using balanced equations mass to moles calculations. Learn how the mole is defined,. The mass contained in one. Learn how to use the mole to calculate mass, number of molecules,. What Is A Mole Used To Measure In Chemistry.
From sciencenotes.org
What Is a Mole In Chemistry? Definition What Is A Mole Used To Measure In Chemistry Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. Mole calculations using balanced equations mass to moles calculations. A mole is a unit of quantity that describes the number of particles. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT Chemistry Calculations PowerPoint Presentation, free download What Is A Mole Used To Measure In Chemistry A mole is a unit of quantity that describes the number of particles in a system. The mass contained in one. Learn how to use the mole to calculate mass, number of molecules, and molar mass. Chemical amounts are measured in moles. Learn how the mole is defined,. Mole calculations using balanced equations mass to moles calculations. Learn how to. What Is A Mole Used To Measure In Chemistry.
From sciencenotes.org
Mole Ratio Definition and Examples What Is A Mole Used To Measure In Chemistry A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Learn how the mole is defined,. The mass contained in one. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. Learn how to calculate the mole, mass, and molecular weight of a. Learn how to. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT 10.1 Mole a Measurement of Matter PowerPoint Presentation, free What Is A Mole Used To Measure In Chemistry Learn how the mole is defined,. Mole calculations using balanced equations mass to moles calculations. Chemical amounts are measured in moles. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. The mass contained in one. A mole is a unit of quantity that describes the number of particles in a system.. What Is A Mole Used To Measure In Chemistry.
From www.pinterest.fr
Chemistry Mole Conversion Chart Images & Pictures Becuo Chemistry What Is A Mole Used To Measure In Chemistry Learn how to use the mole to calculate mass, number of molecules, and molar mass. Learn how to calculate the mole, mass, and molecular weight of a. A mole is a unit of quantity that describes the number of particles in a system. The mass contained in one. A mole is a unit of measurement that represents the amount of. What Is A Mole Used To Measure In Chemistry.
From www.youtube.com
What is a Mole and How to Use the Mole in Chemistry YouTube What Is A Mole Used To Measure In Chemistry Mole calculations using balanced equations mass to moles calculations. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. Learn how the mole is defined,. Learn how to use the mole to calculate mass, number of molecules, and molar mass. The mass contained in one. A mole is a unit. What Is A Mole Used To Measure In Chemistry.
From www.youtube.com
Understanding Moles (GCSE Chemistry) YouTube What Is A Mole Used To Measure In Chemistry A mole is a unit of quantity that describes the number of particles in a system. Learn how to calculate the mole, mass, and molecular weight of a. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. A mole is a unit of measurement for counting atoms, molecules, or. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT Section 7.1 The Mole A Measurement of Matter PowerPoint What Is A Mole Used To Measure In Chemistry Mole calculations using balanced equations mass to moles calculations. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how the mole is defined,. Chemical amounts are measured in moles. Learn how to calculate moles, molar mass, and. A mole is a unit of quantity that describes the number of particles in a system.. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT The Mole A Measurement of Matter PowerPoint Presentation, free What Is A Mole Used To Measure In Chemistry Mole calculations using balanced equations mass to moles calculations. Learn how to use the mole concept and molar mass to calculate the amount of substance in a chemical reaction. Learn how to calculate moles, molar mass, and. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Learn how to calculate the mole, mass,. What Is A Mole Used To Measure In Chemistry.
From surfguppy.com
Mole Archives Surfguppy Chemistry made easy visual learning What Is A Mole Used To Measure In Chemistry Chemical amounts are measured in moles. Mole calculations using balanced equations mass to moles calculations. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how the mole is defined,. Learn how to calculate moles, molar mass, and. Learn how to calculate the mole, mass, and molecular weight of a. A mole is a. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free What Is A Mole Used To Measure In Chemistry A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023 particles. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Learn how to calculate moles, molar mass, and. Mole calculations using. What Is A Mole Used To Measure In Chemistry.
From mungfali.com
The Mole Chemistry What Is A Mole Used To Measure In Chemistry Learn how to use the mole to calculate mass, number of molecules, and molar mass. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Mole calculations using balanced equations mass to moles calculations. Learn how to calculate the mole, mass, and molecular weight of a. A mole is a unit of quantity that. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT The mole PowerPoint Presentation, free download ID1836804 What Is A Mole Used To Measure In Chemistry Chemical amounts are measured in moles. Learn how to use the mole to calculate mass, number of molecules, and molar mass. A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. Learn how the mole is defined,. A mole is a unit of measurement that represents the amount of substance containing 6.02 × 1023. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT The Mole A Measurement of Matter PowerPoint Presentation, free What Is A Mole Used To Measure In Chemistry Learn how to calculate moles, molar mass, and. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to calculate the mole, mass, and molecular weight of a. Mole calculations using balanced equations mass to moles calculations. A mole is a unit of measurement that represents the amount of substance containing 6.02 ×. What Is A Mole Used To Measure In Chemistry.
From www.slideserve.com
PPT The Mole PowerPoint Presentation, free download ID5684599 What Is A Mole Used To Measure In Chemistry A mole is a standard unit for measuring large quantities of atoms, molecules, or other particles. The mass contained in one. A mole is a unit of measurement for counting atoms, molecules, or ions in chemistry. Learn how to use the mole to calculate mass, number of molecules, and molar mass. Learn how to calculate moles, molar mass, and. A. What Is A Mole Used To Measure In Chemistry.
From www.youtube.com
What is a Chemistry Mole....Explained! YouTube What Is A Mole Used To Measure In Chemistry Chemical amounts are measured in moles. Learn how to calculate moles, molar mass, and. Learn how to calculate the mole, mass, and molecular weight of a. Learn how to use the mole to calculate mass, number of molecules, and molar mass. Learn how the mole is defined,. A mole is a unit of measurement for counting atoms, molecules, or ions. What Is A Mole Used To Measure In Chemistry.