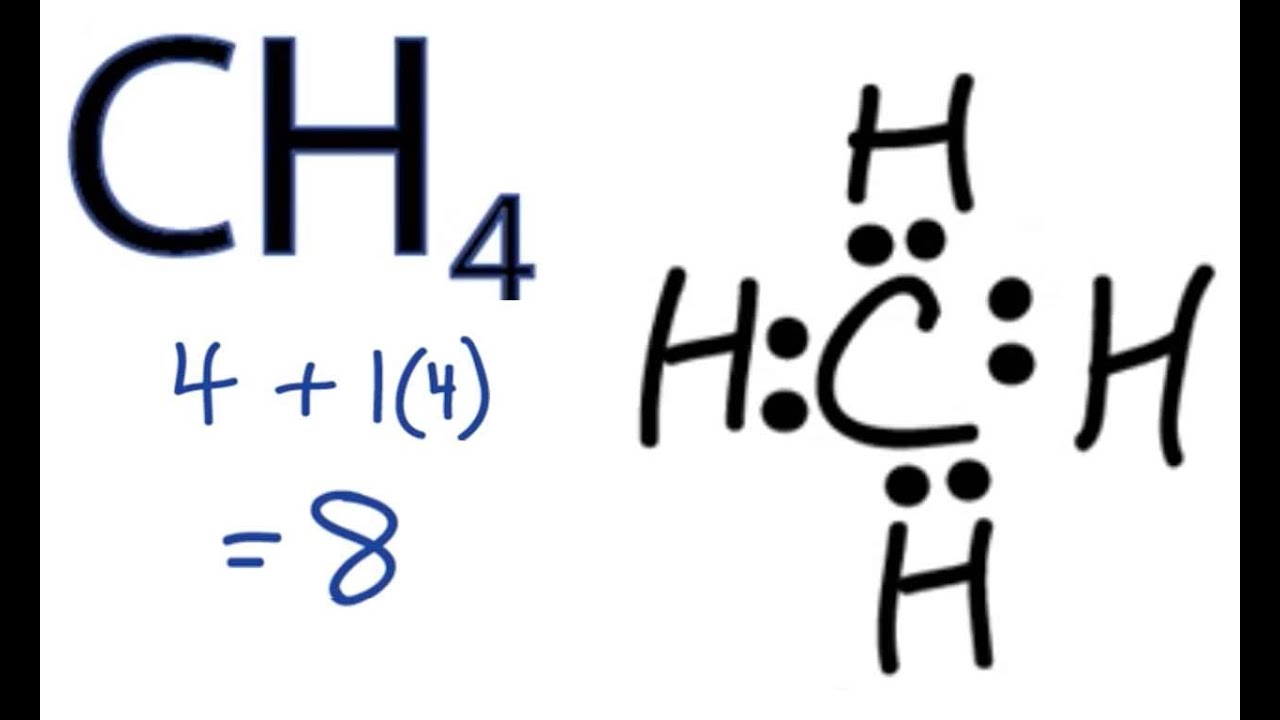What Molecular Shape Is Ch4 . A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,.
from www.youtube.com
A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,.
CH4 Lewis Structure How to Draw the Dot Structure for CH4 (Methane
What Molecular Shape Is Ch4 A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule.
From ar.inspiredpencil.com
Ch4 Lewis Structure Shape What Molecular Shape Is Ch4 Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A molecule with four electron groups about the central atom orients the four groups in the. What Molecular Shape Is Ch4.
From techiescientist.com
CH4 Lewis Structure, Molecular Geometry, and Hybridization What Molecular Shape Is Ch4 Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Let us look at the lewis structure of ch 4. What Molecular Shape Is Ch4.
From ar.inspiredpencil.com
Ch4 Molecule What Molecular Shape Is Ch4 For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. Carbon in methane. What Molecular Shape Is Ch4.
From byjus.com
Methane Formula Structural and Chemical Formula of Methane What Molecular Shape Is Ch4 Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. A molecule with. What Molecular Shape Is Ch4.
From www.youtube.com
CH4O Lewis Structure How to Draw the Lewis Structure for CH4O YouTube What Molecular Shape Is Ch4 Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central.. What Molecular Shape Is Ch4.
From telgurus.co.uk
Explain the molecular structure of CH4 Methane molecular structure What Molecular Shape Is Ch4 A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Carbon in methane takes the. What Molecular Shape Is Ch4.
From www.alamy.com
Molecular Model of Methane, CH4, Illustration Stock Photo Alamy What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. Let us. What Molecular Shape Is Ch4.
From techiescientist.com
CH4 Lewis Structure, Molecular Geometry, and Hybridization What Molecular Shape Is Ch4 Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. A quick explanation. What Molecular Shape Is Ch4.
From www.chemistrylearner.com
Molecular Geometry, Lewis Structure, & Bond Angle of Methane What Molecular Shape Is Ch4 A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Let us look at the lewis structure of ch 4. What Molecular Shape Is Ch4.
From www.alamy.com
Methane (CH4) gas molecule, molecular model. Methane is the main Stock What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the. What Molecular Shape Is Ch4.
From ar.inspiredpencil.com
Ch4 Lewis Structure Shape What Molecular Shape Is Ch4 A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. For example, the methane molecule, ch 4, which is the. What Molecular Shape Is Ch4.
From ar.inspiredpencil.com
Ch4 Molecule Shape What Molecular Shape Is Ch4 A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. For example, the. What Molecular Shape Is Ch4.
From ar.inspiredpencil.com
Ch4 Molecule What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding. What Molecular Shape Is Ch4.
From www.youtube.com
CH4 Lewis Structure How to Draw the Dot Structure for CH4 (Methane What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the. What Molecular Shape Is Ch4.
From www.shutterstock.com
Methane Molecule Ch4 Tetrahedron With Sp3 Hybrid Orbitals Stock What Molecular Shape Is Ch4 A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. A quick. What Molecular Shape Is Ch4.
From www.youtube.com
CH4 Molecular Geometry / Shape and Bond Angles YouTube What Molecular Shape Is Ch4 A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Carbon in methane takes the central. What Molecular Shape Is Ch4.
From www.turbosquid.com
ch4 obj What Molecular Shape Is Ch4 For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Let us. What Molecular Shape Is Ch4.
From sciedutut.com
CH4 Molecular Geometry Science Education and Tutorials What Molecular Shape Is Ch4 For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Carbon in methane takes the central position as it is less electronegative than the hydrogen. What Molecular Shape Is Ch4.
From ar.inspiredpencil.com
Ch4 Molecule What Molecular Shape Is Ch4 A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A molecule with. What Molecular Shape Is Ch4.
From www.vrogue.co
3d Model Molecule Ch4 Royalty Free Vector Image vrogue.co What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in. What Molecular Shape Is Ch4.
From www.alamy.com
Molecular Model of Methane (CH4) Molecule. Vector Illustration Stock What Molecular Shape Is Ch4 A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs. What Molecular Shape Is Ch4.
From mungfali.com
Electron Dot Structure Of CH4 What Molecular Shape Is Ch4 A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. A molecule with. What Molecular Shape Is Ch4.
From imgarcade.com
Gallery For > Ch4 Molecular Shape What Molecular Shape Is Ch4 A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. For example, the methane molecule, ch. What Molecular Shape Is Ch4.
From www.researchgate.net
Schematic diagram of the molecular structure of CH4 Download What Molecular Shape Is Ch4 A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick. What Molecular Shape Is Ch4.
From brainly.in
draw the electron dot structure of CH4 Brainly.in What Molecular Shape Is Ch4 Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Carbon in methane takes the central. What Molecular Shape Is Ch4.
From www.vectorstock.com
3d model molecule ch4 Royalty Free Vector Image What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. A quick explanation. What Molecular Shape Is Ch4.
From www.alamy.com
Methane (CH4) gas molecule, chemical structure. Methane is the main What Molecular Shape Is Ch4 Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. A molecule with. What Molecular Shape Is Ch4.
From ar.inspiredpencil.com
Ch4 Molecular Geometry What Molecular Shape Is Ch4 Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule.. What Molecular Shape Is Ch4.
From scienceinfo.com
Lewis Structure of CH4 What Molecular Shape Is Ch4 For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. Let us. What Molecular Shape Is Ch4.
From www.youtube.com
Molecular Orbital Diagram (MO Diagram) of methane (CH4) Chemical What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick. What Molecular Shape Is Ch4.
From www.pinterest.cl
CH4 Molecular Geometry, Shape and Bond Angles (Methane) in 2023 What Molecular Shape Is Ch4 For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A quick explanation of the molecular geometry for ch4 including bond angle, hybridization,. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick. What Molecular Shape Is Ch4.
From topblogtenz.com
CH4 lewis structure, Molecular geometry, Bond angle, Valence electrons What Molecular Shape Is Ch4 Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in the molecule. A quick explanation. What Molecular Shape Is Ch4.
From www.turbosquid.com
Ch4 molecule methane 3D model TurboSquid 1425569 What Molecular Shape Is Ch4 A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Let us look at the lewis structure of ch 4 and determine how the atoms are arranged in. What Molecular Shape Is Ch4.
From www.pinterest.com
In this video we are going to learn about the Lewis structure of CH4 What Molecular Shape Is Ch4 A quick explanation of the molecular geometry of ch4 including a description of the ch4 bond angles.looking at the ch4 lewis. Carbon in methane takes the central position as it is less electronegative than the hydrogen atoms. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. Let us. What Molecular Shape Is Ch4.
From www.pinterest.dk
Methane, CH4, molecule model and chemical formula. Chemical compound What Molecular Shape Is Ch4 For example, the methane molecule, ch 4, which is the major component of natural gas, has four bonding pairs of electrons around the central. A molecule with four electron groups about the central atom orients the four groups in the direction of a tetrahedron, as. A quick explanation of the molecular geometry of ch4 including a description of the ch4. What Molecular Shape Is Ch4.