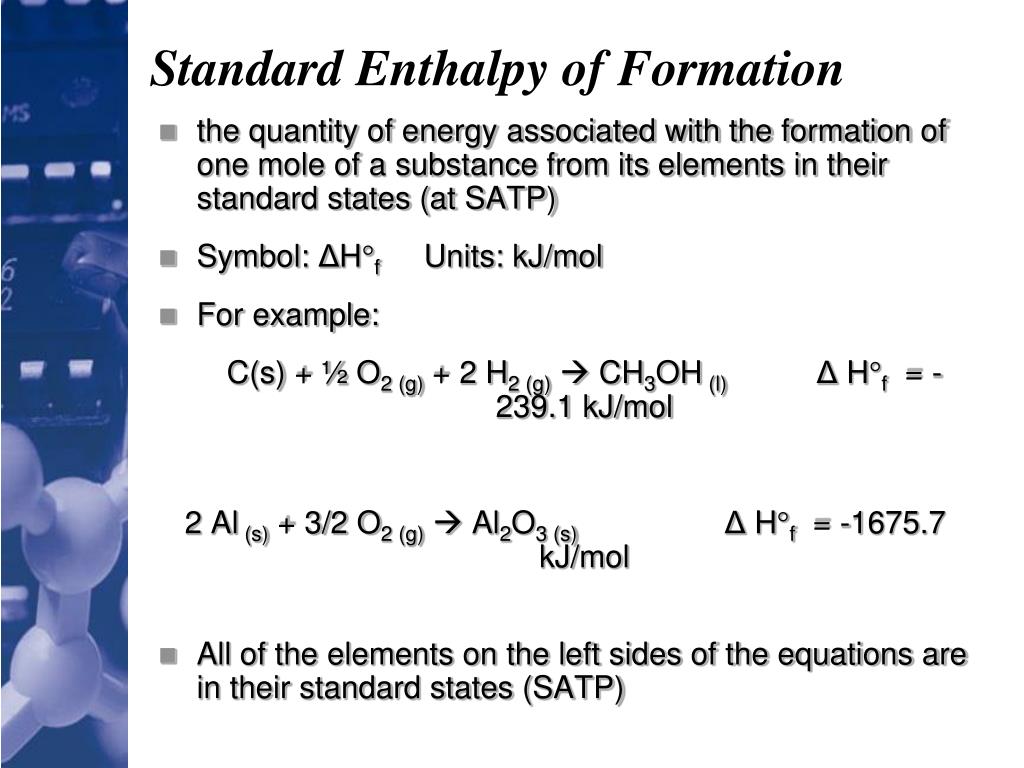
Standard Enthalpy Of Formation Acetic Acid . C 2 h 4 o 2. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. For example, although oxygen can exist as. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5.
from www.slideserve.com
What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. For example, although oxygen can exist as. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5. The standard enthalpy of formation of any element in its standard state is zero by definition.
PPT Standard Enthalpies of Formation PowerPoint Presentation, free
Standard Enthalpy Of Formation Acetic Acid The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. For example, although oxygen can exist as. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 14 rows. Standard Enthalpy Of Formation Acetic Acid.
From fr.wikidoc.org
Acetic acid wikidoc Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. For example, although oxygen can exist as. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. 14 rows selected atct [1, 2] enthalpy of formation based on version. Standard Enthalpy Of Formation Acetic Acid.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. What is the value (use the first one given) for the standard enthalpy of formation, δh f. Standard Enthalpy Of Formation Acetic Acid.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Acetic Acid This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard. Standard Enthalpy Of Formation Acetic Acid.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical. Standard Enthalpy Of Formation Acetic Acid.
From www.youtube.com
Calculate the enthalpy of formation of acetic acid from the following Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. For example, although oxygen can exist as. The standard enthalpy of formation of any element in its standard state is zero by definition. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. C 2 h. Standard Enthalpy Of Formation Acetic Acid.
From www.toppr.com
Calculate the enthalpy of formation of acetic acid (CH3COOH) if its Standard Enthalpy Of Formation Acetic Acid 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. For example, although oxygen can exist as. The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. What is the value (use the first one given) for. Standard Enthalpy Of Formation Acetic Acid.
From askfilo.com
ii) Calculate the enthalpy of formation of acetic acid under the standard.. Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation of any element in its standard state is zero by. Standard Enthalpy Of Formation Acetic Acid.
From byjus.com
26. Heat of dissociation of acetic acid is 0.005 kcal per g hence Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. C 2 h 4 o 2. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although. Standard Enthalpy Of Formation Acetic Acid.
From www.numerade.com
SOLVED Use the standard enthalpies of formation to determine ΔrH* for Standard Enthalpy Of Formation Acetic Acid The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. What is the value (use the first one given) for the standard enthalpy of formation, δh. Standard Enthalpy Of Formation Acetic Acid.
From www.numerade.com
SOLVEDUse the following standard heats of formation to calculate the Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5. For example, although oxygen can exist as. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. What is the value (use the first one given) for the standard enthalpy. Standard Enthalpy Of Formation Acetic Acid.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 14 rows selected atct [1, 2] enthalpy of formation. Standard Enthalpy Of Formation Acetic Acid.
From www.chegg.com
Consider A Reaction Between Acetic Acid And Ethano... Standard Enthalpy Of Formation Acetic Acid The standard enthalpy of formation of any element in its standard state is zero by definition. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. C 2 h 4 o 2. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements. Standard Enthalpy Of Formation Acetic Acid.
From solvedlib.com
Calculate enthalpy for...(a) the formation of acetic … SolvedLib Standard Enthalpy Of Formation Acetic Acid The standard enthalpy of formation of any element in its standard state is zero by definition. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the.. Standard Enthalpy Of Formation Acetic Acid.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Acetic Acid This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. What is. Standard Enthalpy Of Formation Acetic Acid.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Acetic Acid This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. C 2 h 4 o 2. (a) ethyl alcohol,. Standard Enthalpy Of Formation Acetic Acid.
From www.meritnation.com
calculate the enthalpy of formation of acetic acid if its enthalpy of Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: 14 rows. Standard Enthalpy Of Formation Acetic Acid.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. For example, although oxygen can exist as. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard enthalpy of formation of any element in its standard state is zero by definition. This table lists the standard enthalpies (δh°), the free. Standard Enthalpy Of Formation Acetic Acid.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 14 rows. Standard Enthalpy Of Formation Acetic Acid.
From www.doubtnut.com
Calculate the enthalpy of formation of acetic acid (CH(3)COOH) if its Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. C 2 h 4 o 2. For example, although oxygen can exist as. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: (a) ethyl alcohol, c 2 h 5. 14 rows selected atct [1, 2] enthalpy of formation based on version. Standard Enthalpy Of Formation Acetic Acid.
From askfilo.com
Calculate the enthalpy of formation of acetic acid from the following dat.. Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. For example, although oxygen can exist as. 14 rows. Standard Enthalpy Of Formation Acetic Acid.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. What is the. Standard Enthalpy Of Formation Acetic Acid.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as. C 2. Standard Enthalpy Of Formation Acetic Acid.
From brainly.in
Calculate the enthalpy of formation of acetic acid from the following Standard Enthalpy Of Formation Acetic Acid 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. C 2 h 4 o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. (a) ethyl alcohol, c 2 h 5. For example, although oxygen can exist as. C 2 h. Standard Enthalpy Of Formation Acetic Acid.
From www.numerade.com
SOLVED The balanced chemical equation is needed to calculate the Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. For example, although oxygen can exist as. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network. Standard Enthalpy Of Formation Acetic Acid.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy of. Standard Enthalpy Of Formation Acetic Acid.
From www.youtube.com
NUMERICAL Heat of Formation of Acetic Acid YouTube Standard Enthalpy Of Formation Acetic Acid 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as. C 2 h 4 o 2. What is the value (use the first one given) for. Standard Enthalpy Of Formation Acetic Acid.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Acetic Acid For example, although oxygen can exist as. The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: C 2 h 4 o 2. 14 rows selected. Standard Enthalpy Of Formation Acetic Acid.
From www.chm.bris.ac.uk
Acetic Acid Molecule of the Month April 2016 (JSMol version) Standard Enthalpy Of Formation Acetic Acid C 2 h 4 o 2. What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as. This table lists the standard enthalpies (δh°), the free. Standard Enthalpy Of Formation Acetic Acid.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard. Standard Enthalpy Of Formation Acetic Acid.
From brainly.in
calculate the enthalpy of formation of CH3COOH(acetic acid) when Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. The standard enthalpy of formation of any element in its standard state is zero by definition. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. C 2 h 4 o 2. 14 rows selected atct [1, 2] enthalpy of. Standard Enthalpy Of Formation Acetic Acid.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: (a) ethyl alcohol, c 2 h 5. C 2 h 4 o 2. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard. Standard Enthalpy Of Formation Acetic Acid.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Acetic Acid (a) ethyl alcohol, c 2 h 5. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard enthalpy of formation of any element in its standard state is zero by definition. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of. Standard Enthalpy Of Formation Acetic Acid.
From www.youtube.com
Calculate the enthalpy of formation of acetic acid (CH_(3)COOH) if its Standard Enthalpy Of Formation Acetic Acid What is the value (use the first one given) for the standard enthalpy of formation, δh f o, for the following substances: The standard enthalpy of formation of any element in its standard state is zero by definition. 14 rows selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network this version of atct. C. Standard Enthalpy Of Formation Acetic Acid.
From www.researchgate.net
Enthalpies of reaction at 298 K and calculated enthalpies of formation Standard Enthalpy Of Formation Acetic Acid The standard enthalpy of formation of any element in its standard state is zero by definition. C 2 h 4 o 2. C 2 h 4 o 2. (a) ethyl alcohol, c 2 h 5. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. What is. Standard Enthalpy Of Formation Acetic Acid.