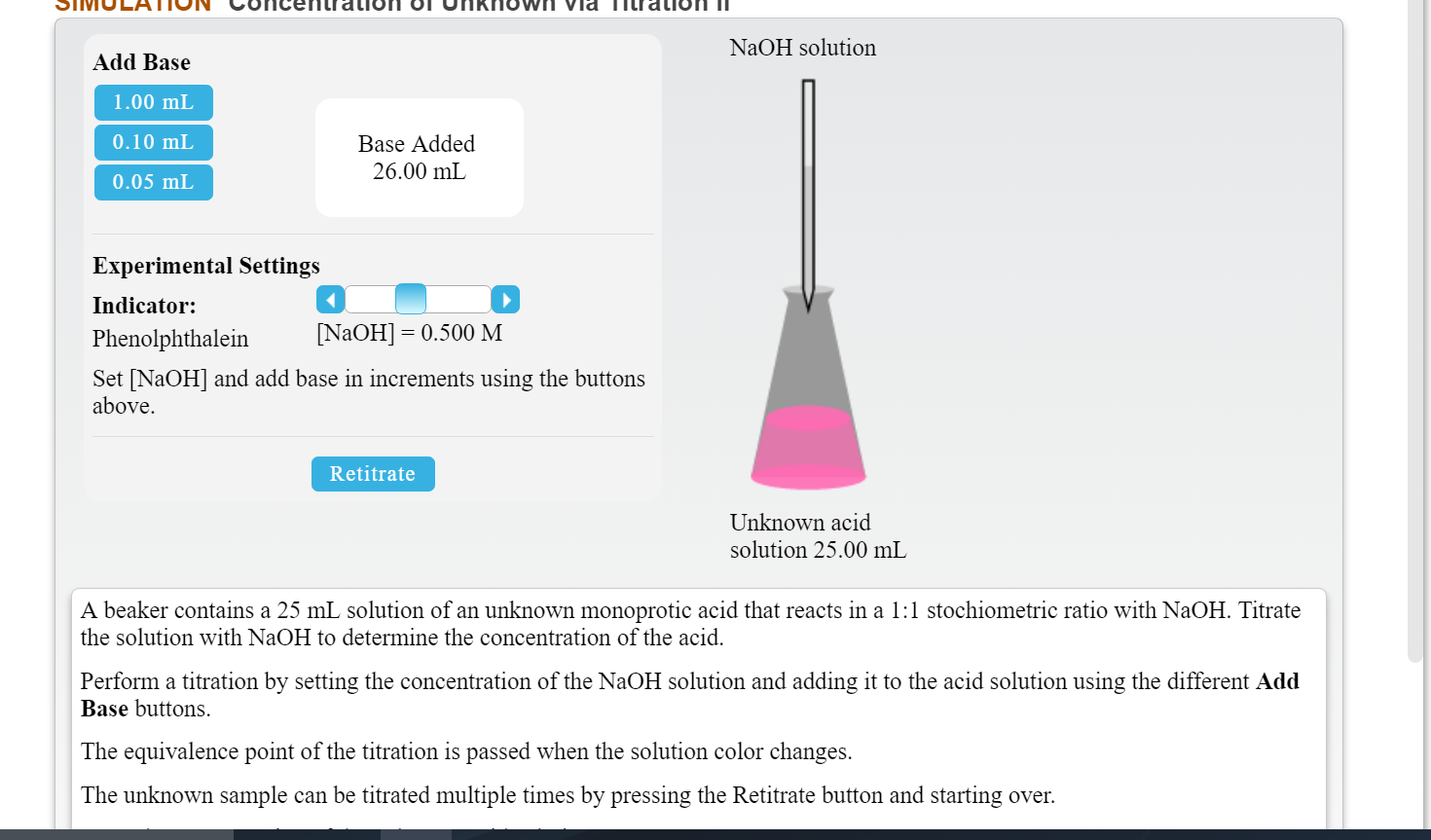
Titration Unknown Concentration . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Calculate the amount of sodium hydroxide in moles. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Suppose that a titration is performed and \(20.70 \: Concordant titres should be used when. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. \ce{naoh}\) is required to reach the end point. The basic process involves adding a standard solution of one reagent to a known amount of the A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3.
from www.chegg.com
A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Concordant titres should be used when. \ce{naoh}\) is required to reach the end point. Suppose that a titration is performed and \(20.70 \: The basic process involves adding a standard solution of one reagent to a known amount of the Calculate the amount of sodium hydroxide in moles.
Solved At the bottom it says " Enter the concentration of
Titration Unknown Concentration Concordant titres should be used when. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Suppose that a titration is performed and \(20.70 \: Concordant titres should be used when. The basic process involves adding a standard solution of one reagent to a known amount of the A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. \ce{naoh}\) is required to reach the end point. Calculate the amount of sodium hydroxide in moles. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Unknown Concentration Calculate the amount of sodium hydroxide in moles. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process involves adding a standard solution of one reagent to a known amount of. Titration Unknown Concentration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Unknown Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Suppose that a titration is performed and \(20.70 \: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 =. Titration Unknown Concentration.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration Unknown Concentration \ce{naoh}\) is required to reach the end point. The basic process involves adding a standard solution of one reagent to a known amount of the Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Volume. Titration Unknown Concentration.
From www.chegg.com
Solved Part II Titration of an acid of unknown concentration Titration Unknown Concentration Concordant titres should be used when. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. The basic process involves adding a standard solution of one reagent to a known amount of the Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. \ce{naoh}\). Titration Unknown Concentration.
From www.chegg.com
Solved Part 2 Titration of Unknown Concentration of Titration Unknown Concentration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Suppose that a. Titration Unknown Concentration.
From www.youtube.com
The titration of 80 0 mL of an unknown concentration of H3PO4 solution Titration Unknown Concentration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Suppose that a titration is performed and \(20.70 \: Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. A titration is a technique where a solution of known concentration is used to determine the concentration. Titration Unknown Concentration.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d Titration Unknown Concentration Concordant titres should be used when. Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration Unknown Concentration.
From www.numerade.com
SOLVED Determine the concentration of the unknown acid using the above Titration Unknown Concentration The basic process involves adding a standard solution of one reagent to a known amount of the Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration Unknown Concentration.
From www.slideserve.com
PPT AcidBase Titrations A. Definition volumetric analysis of the Titration Unknown Concentration Concordant titres should be used when. Calculate the amount of sodium hydroxide in moles. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. \ce{naoh}\) is required to reach the end point. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is used to. Titration Unknown Concentration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution Titration Unknown Concentration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Data collected during a titration allows chemists to determine a. Titration Unknown Concentration.
From dokumen.tips
(PPT) Acid Base Titrations Chemistry 12 Chapter 14. Titration A Titration Unknown Concentration Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Calculate the amount of sodium hydroxide in moles. Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the slow addition. Titration Unknown Concentration.
From www.youtube.com
CHEM 101 Titration of Unknown Triprotic Acid YouTube Titration Unknown Concentration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point. Calculate the amount of sodium hydroxide in moles.. Titration Unknown Concentration.
From www.chegg.com
Solved 8. The titration of 25.0 mL of an unknown Titration Unknown Concentration Suppose that a titration is performed and \(20.70 \: The basic process involves adding a standard solution of one reagent to a known amount of the Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. \ce{naoh}\) is required to reach the end point. A titration is a technique where a solution of known concentration is used. Titration Unknown Concentration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration Unknown Concentration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Concordant titres should be used when. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. \ce{naoh}\) is required to reach the end point. Volume of. Titration Unknown Concentration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Unknown Concentration The basic process involves adding a standard solution of one reagent to a known amount of the Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of. Titration Unknown Concentration.
From teoriadelconocimiento.com.ar
Solved PART TWO TITRATION OF AN UNKNOWN HYDROCHLORIC ACID, 60 OFF Titration Unknown Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. \ce{naoh}\) is required to reach the end point. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Concordant titres should be used when. A titration is used to find an unknown concentration of a solution by. Titration Unknown Concentration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Unknown Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of.. Titration Unknown Concentration.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A Titration Unknown Concentration Suppose that a titration is performed and \(20.70 \: The basic process involves adding a standard solution of one reagent to a known amount of the Calculate the amount of sodium hydroxide in moles. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Concordant titres should be used. Titration Unknown Concentration.
From slideplayer.com
Titration. ppt download Titration Unknown Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a standard solution of one reagent to a known amount of the \ce{naoh}\) is required to reach the end point. Concordant titres should be used when. Data collected during a titration allows chemists to determine. Titration Unknown Concentration.
From www.youtube.com
titration solving for an unknown concentration YouTube Titration Unknown Concentration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. \ce{naoh}\) is required to reach the end point. Suppose that a titration is performed and \(20.70 \: The. Titration Unknown Concentration.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Unknown Concentration Calculate the amount of sodium hydroxide in moles. Suppose that a titration is performed and \(20.70 \: Concordant titres should be used when. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The. Titration Unknown Concentration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Unknown Concentration The basic process involves adding a standard solution of one reagent to a known amount of the Data collected during a titration allows chemists to determine a solution’s unknown concentration. Concordant titres should be used when. Suppose that a titration is performed and \(20.70 \: A titration is used to find an unknown concentration of a solution by reacting it. Titration Unknown Concentration.
From www.vrogue.co
What Is Titration Multiple Choice A A Solution Of Kno vrogue.co Titration Unknown Concentration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Calculate the amount of sodium hydroxide in moles. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Suppose that a titration is performed and \(20.70 \: The basic process involves adding a standard solution of. Titration Unknown Concentration.
From brainly.com
In a titration of a solution of unknown concentration of calcium Titration Unknown Concentration A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. The basic process involves adding a standard solution of one reagent to a known amount of the Suppose that a titration is performed and \(20.70 \: A titration is a laboratory technique used to precisely measure molar concentration of. Titration Unknown Concentration.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Titration Unknown Concentration Calculate the amount of sodium hydroxide in moles. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. \ce{naoh}\) is required to reach the end point. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Volume of. Titration Unknown Concentration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Unknown Concentration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Concordant titres should be used when. Calculate the amount of sodium hydroxide in moles. Data collected during a titration allows chemists to determine a solution’s unknown concentration. A titration is used to find an unknown concentration of a solution. Titration Unknown Concentration.
From www.numerade.com
SOLVED A student carried out two titrations using a NaOH solution of Titration Unknown Concentration The basic process involves adding a standard solution of one reagent to a known amount of the Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration Unknown Concentration.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Unknown Concentration Calculate the amount of sodium hydroxide in moles. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Suppose that a titration is performed and \(20.70 \: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is the slow addition of one solution of. Titration Unknown Concentration.
From freeonlinehelpns.blogspot.com
Free Online Help QThe titration of 10.00 mL of a diprotic acid Titration Unknown Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. The basic process involves adding a standard solution of one reagent to a known amount of the Concordant titres. Titration Unknown Concentration.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Titration Unknown Concentration Concordant titres should be used when. Calculate the amount of sodium hydroxide in moles. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. \ce{naoh}\) is required to reach the end point. A titration is a. Titration Unknown Concentration.
From www.chegg.com
UNKNOWN TITRATION Unknown 25 mLFe2+ unknown Average Titration Unknown Concentration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Concordant titres should be used when. Suppose that a titration is performed and \(20.70 \: The basic process involves adding a standard solution of one reagent to a known amount of the A titration is used to. Titration Unknown Concentration.
From mungfali.com
Acid Base Titration Calculation Titration Unknown Concentration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point. A titration is used to find an unknown. Titration Unknown Concentration.
From www.chegg.com
Solved At the bottom it says " Enter the concentration of Titration Unknown Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Concordant titres should be used when. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution.. Titration Unknown Concentration.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Unknown Concentration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Calculate the amount of sodium hydroxide in moles. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Suppose that a titration is performed and \(20.70. Titration Unknown Concentration.
From slideplayer.com
Volumetric Analysis Using titration of a solution with known Titration Unknown Concentration Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250 dm 3. Calculate the amount of sodium hydroxide in moles. A titration is used to find an unknown concentration of a solution by reacting it with a solution of known concentration. Suppose that a titration is performed and \(20.70 \: A titration is a technique where a solution of. Titration Unknown Concentration.