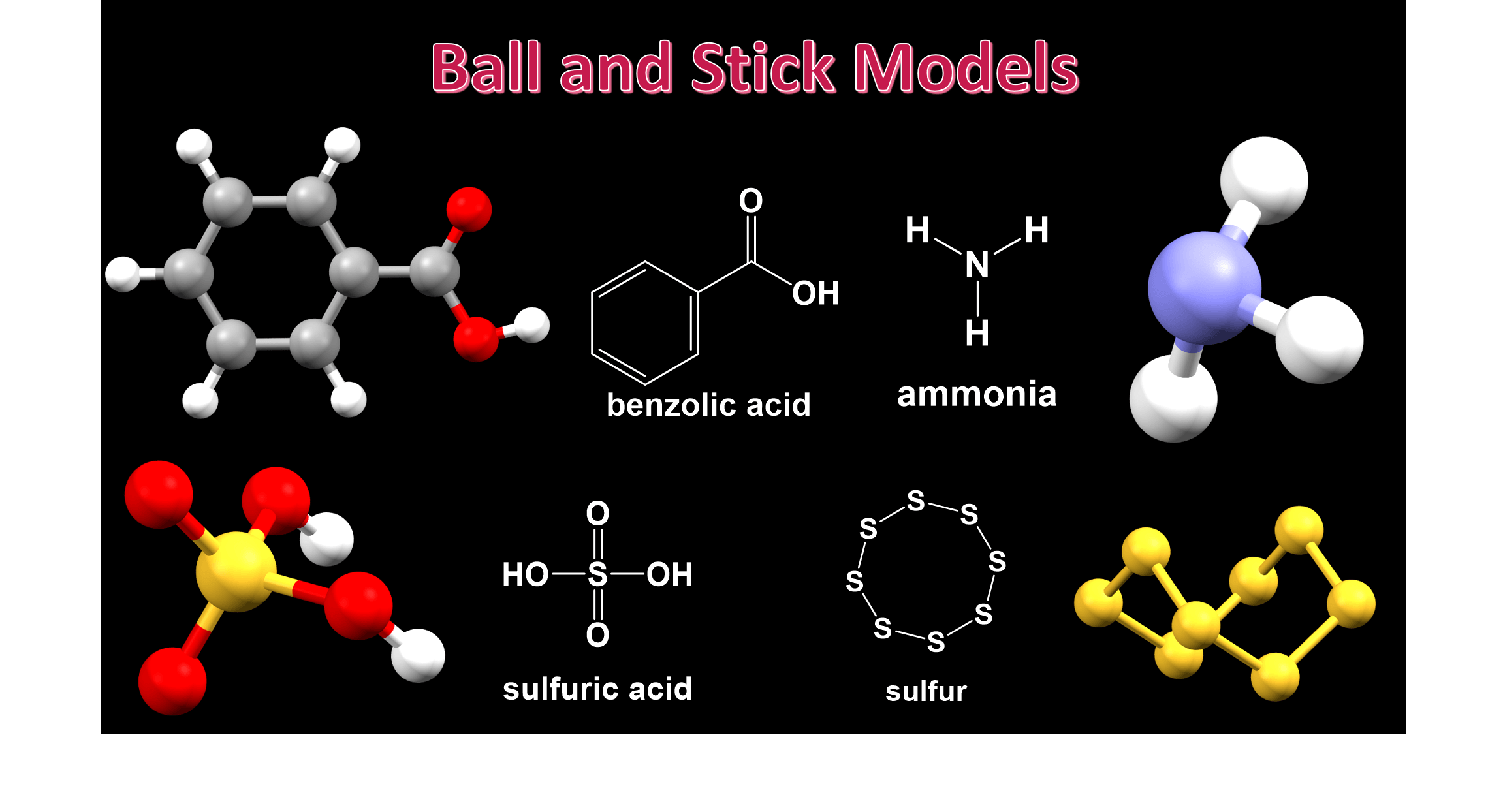
Molecular Models With Single Bonds . Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. So unless a detailed picture of molecular structure is required, one of the simpler models is used. Comment on the distinction between a theory and a model in the context of chemical bonding. Pick one of the bond types (single, double, triple, up, down) and add. This exercise will give you practice in using lewis dot structures,. Explore molecule shapes by building molecules in 3d! Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. Converts the structural formula into a 3d model. Find out by adding single, double or triple bonds. What is meant by a classical model of chemical bonding? How does molecule shape change with different numbers of bonds and electron pairs? An interactive simulation by phet that allows users to build molecules and understand their structures.
from www.expii.com
So unless a detailed picture of molecular structure is required, one of the simpler models is used. What is meant by a classical model of chemical bonding? An interactive simulation by phet that allows users to build molecules and understand their structures. Converts the structural formula into a 3d model. Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. Pick one of the bond types (single, double, triple, up, down) and add. Explore molecule shapes by building molecules in 3d! Comment on the distinction between a theory and a model in the context of chemical bonding. How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds.
Molecular Models — BallandStick Model & SpaceFilling Model Expii
Molecular Models With Single Bonds How does molecule shape change with different numbers of bonds and electron pairs? Pick one of the bond types (single, double, triple, up, down) and add. Comment on the distinction between a theory and a model in the context of chemical bonding. This exercise will give you practice in using lewis dot structures,. So unless a detailed picture of molecular structure is required, one of the simpler models is used. How does molecule shape change with different numbers of bonds and electron pairs? Explore molecule shapes by building molecules in 3d! An interactive simulation by phet that allows users to build molecules and understand their structures. Find out by adding single, double or triple bonds. What is meant by a classical model of chemical bonding? Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. Converts the structural formula into a 3d model.
From courses.lumenlearning.com
Molecular Structure and Polarity Chemistry for Majors Molecular Models With Single Bonds An interactive simulation by phet that allows users to build molecules and understand their structures. How does molecule shape change with different numbers of bonds and electron pairs? What is meant by a classical model of chemical bonding? Find out by adding single, double or triple bonds. Converts the structural formula into a 3d model. Molecular models are designed to. Molecular Models With Single Bonds.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica Molecular Models With Single Bonds What is meant by a classical model of chemical bonding? So unless a detailed picture of molecular structure is required, one of the simpler models is used. Find out by adding single, double or triple bonds. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. Pick one of the bond. Molecular Models With Single Bonds.
From www.britannica.com
Chemical bonding Molecular Shapes, VSEPR Theory Britannica Molecular Models With Single Bonds Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. So unless a detailed picture of molecular structure is required, one of the simpler models is used. How does molecule shape change with different numbers of bonds and electron pairs? Molecular models can help with obtaining the correct lewis structure since the. Molecular Models With Single Bonds.
From www.chegg.com
PRELABORATORY ASSIGNMENT 1. In your own words, define Molecular Models With Single Bonds Pick one of the bond types (single, double, triple, up, down) and add. Explore molecule shapes by building molecules in 3d! Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. Converts the structural formula into a 3d model. How does molecule shape change with different numbers of bonds and electron pairs?. Molecular Models With Single Bonds.
From courses.lumenlearning.com
Molecular Geometry Boundless Chemistry Molecular Models With Single Bonds So unless a detailed picture of molecular structure is required, one of the simpler models is used. Explore molecule shapes by building molecules in 3d! Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. How does molecule shape change with different numbers of bonds and electron pairs? An interactive simulation. Molecular Models With Single Bonds.
From kaiserscience.wordpress.com
Molecular geometry gumdrop lab « KaiserScience Molecular Models With Single Bonds Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. How does molecule shape change with different numbers of bonds and electron pairs? Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. An interactive simulation by phet that allows users to build. Molecular Models With Single Bonds.
From www.xaktly.com
VSEPR theory Molecular Models With Single Bonds This exercise will give you practice in using lewis dot structures,. An interactive simulation by phet that allows users to build molecules and understand their structures. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. Pick one of the bond types (single, double, triple, up, down) and add. Comment on. Molecular Models With Single Bonds.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules Molecular Models With Single Bonds What is meant by a classical model of chemical bonding? Converts the structural formula into a 3d model. Explore molecule shapes by building molecules in 3d! An interactive simulation by phet that allows users to build molecules and understand their structures. Find out by adding single, double or triple bonds. So unless a detailed picture of molecular structure is required,. Molecular Models With Single Bonds.
From study.com
VSEPR Theory Chart & Model Lesson Molecular Models With Single Bonds So unless a detailed picture of molecular structure is required, one of the simpler models is used. Pick one of the bond types (single, double, triple, up, down) and add. How does molecule shape change with different numbers of bonds and electron pairs? Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes. Molecular Models With Single Bonds.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules Molecular Models With Single Bonds Explore molecule shapes by building molecules in 3d! This exercise will give you practice in using lewis dot structures,. What is meant by a classical model of chemical bonding? So unless a detailed picture of molecular structure is required, one of the simpler models is used. Molecular models can help with obtaining the correct lewis structure since the bond positions. Molecular Models With Single Bonds.
From matteoportfoliorecapchem.weebly.com
Organic Chemistry Molecular Models With Single Bonds Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. Find out by adding single, double or triple bonds. Converts the structural formula into a 3d model. Pick one of the bond types (single, double, triple, up, down) and add. How does molecule shape change with different numbers of bonds and electron. Molecular Models With Single Bonds.
From www.expii.com
Hydrogen Bonds — Overview & Examples Expii Molecular Models With Single Bonds This exercise will give you practice in using lewis dot structures,. Converts the structural formula into a 3d model. An interactive simulation by phet that allows users to build molecules and understand their structures. So unless a detailed picture of molecular structure is required, one of the simpler models is used. Molecular models can help with obtaining the correct lewis. Molecular Models With Single Bonds.
From www.pinterest.com
Introduction to Molecular bonding and VSEPR Theory Lets Learn Nepal Molecular Models With Single Bonds So unless a detailed picture of molecular structure is required, one of the simpler models is used. How does molecule shape change with different numbers of bonds and electron pairs? Pick one of the bond types (single, double, triple, up, down) and add. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom. Molecular Models With Single Bonds.
From saylordotorg.github.io
Naming Covalent Compounds Molecular Models With Single Bonds An interactive simulation by phet that allows users to build molecules and understand their structures. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. So unless a detailed picture of molecular structure is required, one of the simpler models is used. Find out by adding single, double or triple bonds.. Molecular Models With Single Bonds.
From www.coolkidfacts.com
Chemical Bonding Facts Chemistry Cool Kid Facts Molecular Models With Single Bonds Find out by adding single, double or triple bonds. How does molecule shape change with different numbers of bonds and electron pairs? This exercise will give you practice in using lewis dot structures,. Comment on the distinction between a theory and a model in the context of chemical bonding. So unless a detailed picture of molecular structure is required, one. Molecular Models With Single Bonds.
From general.chemistrysteps.com
VSEPR Theory Chemistry Steps Molecular Models With Single Bonds This exercise will give you practice in using lewis dot structures,. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. Find out by adding single, double or triple bonds. Pick one of the bond types (single, double, triple, up, down) and add. What is meant by a classical model of. Molecular Models With Single Bonds.
From chemsjc.blogspot.com
Jimchem VSEPR Theory Molecular Models With Single Bonds Converts the structural formula into a 3d model. Explore molecule shapes by building molecules in 3d! What is meant by a classical model of chemical bonding? So unless a detailed picture of molecular structure is required, one of the simpler models is used. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom. Molecular Models With Single Bonds.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules Molecular Models With Single Bonds How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds. So unless a detailed picture of molecular structure is required, one of the simpler models is used. What is meant by a classical model of chemical bonding? An interactive simulation by phet that allows users to build molecules. Molecular Models With Single Bonds.
From chem.libretexts.org
3.1 Types of Chemical Compounds and their Formulas Chemistry LibreTexts Molecular Models With Single Bonds Explore molecule shapes by building molecules in 3d! Comment on the distinction between a theory and a model in the context of chemical bonding. Pick one of the bond types (single, double, triple, up, down) and add. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. How does molecule shape. Molecular Models With Single Bonds.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Molecular Models With Single Bonds Find out by adding single, double or triple bonds. So unless a detailed picture of molecular structure is required, one of the simpler models is used. Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. Comment on the distinction between a theory and a model in the context of chemical bonding.. Molecular Models With Single Bonds.
From www.thoughtco.com
Bonds Definition and Examples in Chemistry Molecular Models With Single Bonds Converts the structural formula into a 3d model. Explore molecule shapes by building molecules in 3d! What is meant by a classical model of chemical bonding? This exercise will give you practice in using lewis dot structures,. Find out by adding single, double or triple bonds. How does molecule shape change with different numbers of bonds and electron pairs? Molecular. Molecular Models With Single Bonds.
From courses.lumenlearning.com
Molecular Structure and Polarity Chemistry Molecular Models With Single Bonds Converts the structural formula into a 3d model. What is meant by a classical model of chemical bonding? Find out by adding single, double or triple bonds. This exercise will give you practice in using lewis dot structures,. So unless a detailed picture of molecular structure is required, one of the simpler models is used. Molecular models can help with. Molecular Models With Single Bonds.
From geometrytipstekhnik.blogspot.com
23+ Molecular Geometry Chart With Bond Angles Image GM Molecular Models With Single Bonds An interactive simulation by phet that allows users to build molecules and understand their structures. Explore molecule shapes by building molecules in 3d! Comment on the distinction between a theory and a model in the context of chemical bonding. Converts the structural formula into a 3d model. How does molecule shape change with different numbers of bonds and electron pairs?. Molecular Models With Single Bonds.
From courses.lumenlearning.com
Hydrocarbons CHEM 1305 General Chemistry I—Lecture Molecular Models With Single Bonds An interactive simulation by phet that allows users to build molecules and understand their structures. Comment on the distinction between a theory and a model in the context of chemical bonding. Explore molecule shapes by building molecules in 3d! Converts the structural formula into a 3d model. Molecular models can help with obtaining the correct lewis structure since the bond. Molecular Models With Single Bonds.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Molecular Models With Single Bonds Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. An interactive simulation by phet that allows users to build molecules and understand their structures. Pick one of the bond types (single, double, triple, up, down) and add. Comment on the distinction between a theory and a model in the context of. Molecular Models With Single Bonds.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Molecular Models With Single Bonds Molecular models are designed to reproduce molecular structures in three dimensions, allowing many subtle features concerning shapes of. What is meant by a classical model of chemical bonding? So unless a detailed picture of molecular structure is required, one of the simpler models is used. Comment on the distinction between a theory and a model in the context of chemical. Molecular Models With Single Bonds.
From www.chegg.com
Solved A. Molecular Models with Single Bonds Model Kit Molecular Models With Single Bonds Pick one of the bond types (single, double, triple, up, down) and add. What is meant by a classical model of chemical bonding? This exercise will give you practice in using lewis dot structures,. An interactive simulation by phet that allows users to build molecules and understand their structures. Explore molecule shapes by building molecules in 3d! So unless a. Molecular Models With Single Bonds.
From cartridges.esciencelabs.com
Diagram of shapes of molecules, showng bonding pairs, arrangement of Molecular Models With Single Bonds Pick one of the bond types (single, double, triple, up, down) and add. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. This exercise will give you practice in using lewis dot structures,. What is meant by a classical model of chemical bonding? How does molecule shape change with different. Molecular Models With Single Bonds.
From www.pinterest.com
VSEPR theory and molecular shapes Molecular geometry, Science Molecular Models With Single Bonds Converts the structural formula into a 3d model. What is meant by a classical model of chemical bonding? Pick one of the bond types (single, double, triple, up, down) and add. How does molecule shape change with different numbers of bonds and electron pairs? This exercise will give you practice in using lewis dot structures,. An interactive simulation by phet. Molecular Models With Single Bonds.
From mavink.com
Covalent Bond Types Molecular Models With Single Bonds Comment on the distinction between a theory and a model in the context of chemical bonding. This exercise will give you practice in using lewis dot structures,. Explore molecule shapes by building molecules in 3d! Find out by adding single, double or triple bonds. Molecular models can help with obtaining the correct lewis structure since the bond positions for each. Molecular Models With Single Bonds.
From www.walmart.com
Chemistry Molecular Model Kit Dioxide Carbon Atoms with Single Bonds Molecular Models With Single Bonds What is meant by a classical model of chemical bonding? So unless a detailed picture of molecular structure is required, one of the simpler models is used. Find out by adding single, double or triple bonds. How does molecule shape change with different numbers of bonds and electron pairs? Explore molecule shapes by building molecules in 3d! Comment on the. Molecular Models With Single Bonds.
From www.chemistrylearner.com
Chemical Bonds Definition, Types, and Examples Molecular Models With Single Bonds This exercise will give you practice in using lewis dot structures,. Converts the structural formula into a 3d model. So unless a detailed picture of molecular structure is required, one of the simpler models is used. Find out by adding single, double or triple bonds. What is meant by a classical model of chemical bonding? How does molecule shape change. Molecular Models With Single Bonds.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Molecular Models With Single Bonds Find out by adding single, double or triple bonds. An interactive simulation by phet that allows users to build molecules and understand their structures. Molecular models can help with obtaining the correct lewis structure since the bond positions for each atom are indicated. Pick one of the bond types (single, double, triple, up, down) and add. So unless a detailed. Molecular Models With Single Bonds.
From chem.libretexts.org
11.6 Delocalized Electrons Bonding in the Benzene Molecule Molecular Models With Single Bonds So unless a detailed picture of molecular structure is required, one of the simpler models is used. This exercise will give you practice in using lewis dot structures,. Pick one of the bond types (single, double, triple, up, down) and add. Find out by adding single, double or triple bonds. Molecular models can help with obtaining the correct lewis structure. Molecular Models With Single Bonds.
From www.expii.com
Molecular Models — BallandStick Model & SpaceFilling Model Expii Molecular Models With Single Bonds Explore molecule shapes by building molecules in 3d! Comment on the distinction between a theory and a model in the context of chemical bonding. So unless a detailed picture of molecular structure is required, one of the simpler models is used. This exercise will give you practice in using lewis dot structures,. Molecular models are designed to reproduce molecular structures. Molecular Models With Single Bonds.