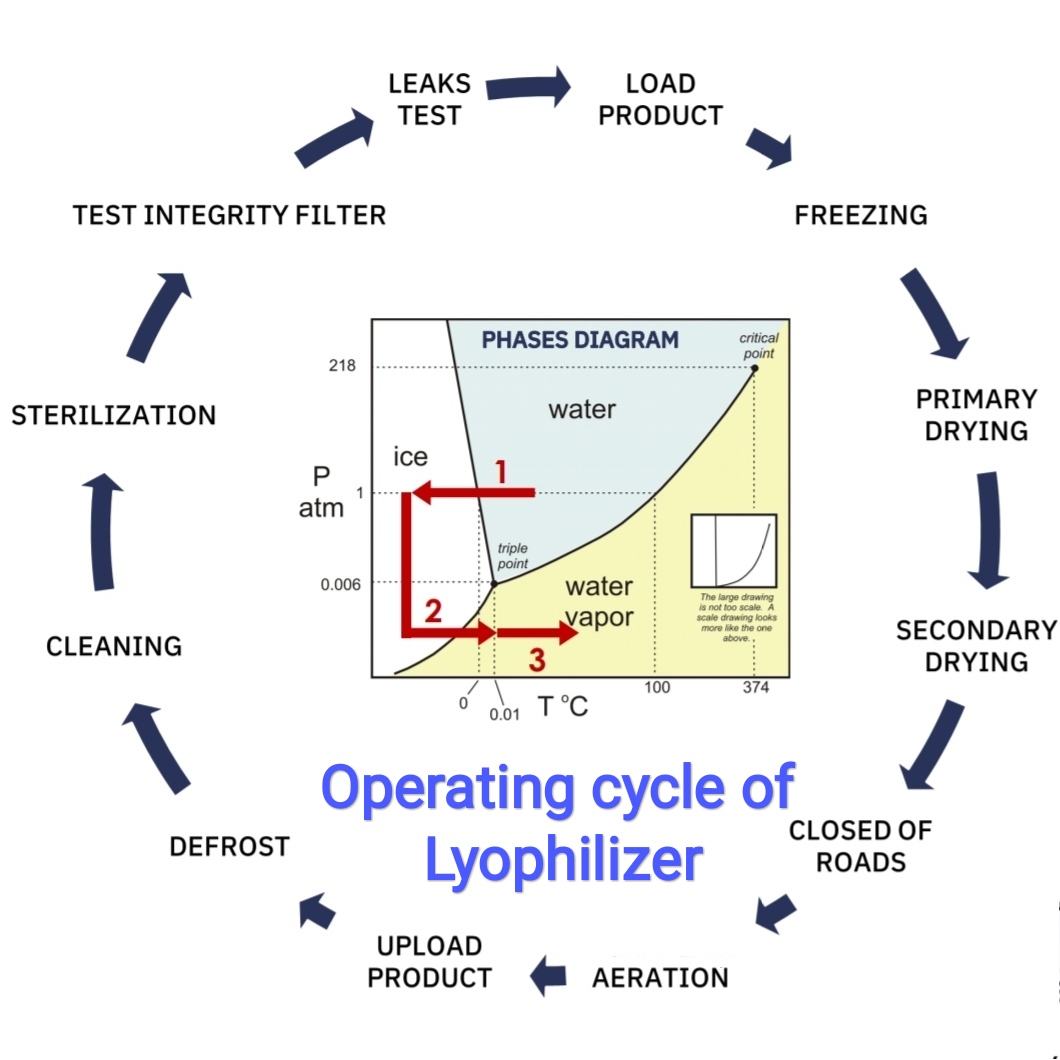
Lyophilization Parameters . This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The validation activities of pharmaceutical lyophilization for stage 1. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key.
from insideofpharma.blogspot.com
Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The validation activities of pharmaceutical lyophilization for stage 1. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary.
Pharma Knowledge Lyophilization or Freeze Drying
Lyophilization Parameters Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The validation activities of pharmaceutical lyophilization for stage 1. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low.
From www.slideserve.com
PPT Principles and Applications of Lyophilization to Biotechnology Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. This study presents best practices for batch size determination and includes the effect of batch size on drying. Lyophilization Parameters.
From pharmagxp.com
Lyophilization or FreezeDrying The Definitive Guide Pharma GxP Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2. Lyophilization Parameters.
From www.scribd.com
Optimizing the Lyophilization Process A Guide to Determining Critical Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure,. Lyophilization Parameters.
From www.slideshare.net
Lyophilization Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,.. Lyophilization Parameters.
From www.mdpi.com
Pharmaceutics Free FullText Lyophilization of Nanocapsules Lyophilization Parameters The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. Lyophilization cycle parameters are optimized for. Lyophilization Parameters.
From www.researchgate.net
Phase diagram illustrating the lyophilization process in detail. Figure Lyophilization Parameters The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. This study presents best practices for batch size determination and includes the effect of. Lyophilization Parameters.
From www.researchgate.net
Critical parameter of lyophilization process. Download Table Lyophilization Parameters Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The validation activities of pharmaceutical lyophilization for stage 1. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure,. Lyophilization Parameters.
From www.slideshare.net
Lyophilization Lyophilization Parameters This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The critical lyophilization operating parameters (e.g.,. Lyophilization Parameters.
From www.researchgate.net
Steps of Lyophilization Process Pharmaceutical FreezeDrying Equipment Lyophilization Parameters Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The process of lyophilization. Lyophilization Parameters.
From www.pharmamanufacturing.com
Grasping Lyophilization Product and Process Parameters for Biopharma Lyophilization Parameters Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The. Lyophilization Parameters.
From www.lyophilizationworld.com
The Complexity and Beauty of Lyophilization Lyophilization Parameters The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The validation activities of pharmaceutical lyophilization for stage 1. Lyophilization cycle. Lyophilization Parameters.
From www.academia.edu
(PDF) A QbD Case Study Bayesian Prediction of Lyophilization Cycle Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. Temperature, pressure control, and time. Lyophilization Parameters.
From jpharmsci.org
Impact of Annealing and Controlled Ice Nucleation on Properties of A Lyophilization Parameters The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The validation activities of pharmaceutical lyophilization for stage 1. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Temperature, pressure control, and time. Lyophilization Parameters.
From www.slideshare.net
Lyophilization Lyophilization Parameters This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The validation activities of pharmaceutical lyophilization for stage 1. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. Temperature, pressure control, and time are well recognized as the principal critical process. Lyophilization Parameters.
From jpharmsci.org
Lyophilization Cycle Design for Dual Chamber Cartridges and a Method Lyophilization Parameters Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The validation activities of pharmaceutical lyophilization for stage 1. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. This study presents best practices. Lyophilization Parameters.
From insideofpharma.blogspot.com
Pharma Knowledge Lyophilization or Freeze Drying Lyophilization Parameters This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The validation activities of pharmaceutical lyophilization for stage 1. Temperature, pressure control, and time are well recognized as the principal critical process. Lyophilization Parameters.
From www.researchgate.net
Lyophilization process diagrams for fullscale engineering batches of Lyophilization Parameters The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The validation activities of pharmaceutical lyophilization for stage 1. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study. Lyophilization Parameters.
From www.researchgate.net
Freezedrying parameters for uncontrolled and controlled nucleation Lyophilization Parameters The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes. Lyophilization Parameters.
From www.semanticscholar.org
Table 4 from Modeling the Effects of Osmotic Dehydration Pretreatment Lyophilization Parameters The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The validation activities of pharmaceutical lyophilization for stage 1. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process. Lyophilization Parameters.
From www.researchgate.net
Lyophilization cycle for freeze drying of raloxifene hydrochloride Lyophilization Parameters The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The process of lyophilization plays a crucial. Lyophilization Parameters.
From researchmethods.web.fc2.com
Lyophilization cycle development ppt presentation researchmethods.web Lyophilization Parameters The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The validation activities of pharmaceutical lyophilization for stage 1. Temperature, pressure control, and time. Lyophilization Parameters.
From www.semanticscholar.org
Figure 1 from Recent Development of Optimization of Lyophilization Lyophilization Parameters The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The process of lyophilization. Lyophilization Parameters.
From www.mdpi.com
Pharmaceutics Free FullText Lyophilization of Nanocapsules Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes the effect of batch size on. Lyophilization Parameters.
From pharmagxp.com
Lyophilization or FreezeDrying The Definitive Guide Pharma GxP Lyophilization Parameters This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Lyophilization cycle parameters are optimized for multiple factors such as a low. Lyophilization Parameters.
From www.labroots.com
The benefits of lyophilization in assay kit development Clinical And Lyophilization Parameters This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The. Lyophilization Parameters.
From www.researchgate.net
(a) Schematic illustration of lyophilization process. Green and red Lyophilization Parameters This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The process of lyophilization. Lyophilization Parameters.
From www.ijpsonline.com
Design of the Lyophilization Process of a Doxorubicin Formulation Based Lyophilization Parameters Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The. Lyophilization Parameters.
From www.researchgate.net
Parameters list used in the regression of the freezedrying parameters Lyophilization Parameters The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The critical lyophilization operating parameters (e.g., shelf temperature, chamber. Lyophilization Parameters.
From pharmagxp.com
Lyophilization or FreezeDrying The Definitive Guide Pharma GxP Lyophilization Parameters The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes. Lyophilization Parameters.
From www.mdpi.com
IJMS Free FullText Lyophilization of Nanoparticles, Does It Really Lyophilization Parameters This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. Temperature, pressure control, and time are well. Lyophilization Parameters.
From www.mdpi.com
Pharmaceutics Free FullText Lyophilization of Nanocapsules Lyophilization Parameters Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. Lyophilization cycle parameters are optimized for multiple factors such as a. Lyophilization Parameters.
From www.slideserve.com
PPT Principles and Applications of Lyophilization to Biotechnology Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Lyophilization cycle parameters. Lyophilization Parameters.
From www.researchgate.net
Critical parameter of lyophilization process. Download Table Lyophilization Parameters The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. This study presents best practices for batch size determination and includes the effect of batch size on drying time, process. Lyophilization cycle parameters are optimized for. Lyophilization Parameters.
From www.researchgate.net
Common PATs used in Lyophilization Process monitoringCM Capacitance Lyophilization Parameters Lyophilization cycle parameters are optimized for multiple factors such as a low residual moisture, cake appearance, reconstitution, low. The validation activities of pharmaceutical lyophilization for stage 1. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. The process of lyophilization plays a crucial part in the manufacturing of pharmaceutical,. This study presents best practices. Lyophilization Parameters.
From www.semanticscholar.org
Table 1 from Modeling the Effects of Osmotic Dehydration Pretreatment Lyophilization Parameters The validation activities of pharmaceutical lyophilization for stage 1. Temperature, pressure control, and time are well recognized as the principal critical process parameters for lyophilization.2 eachcritical process parameters or key. The critical lyophilization operating parameters (e.g., shelf temperature, chamber pressure, annealing time, freezing rate, primary. This study presents best practices for batch size determination and includes the effect of batch. Lyophilization Parameters.