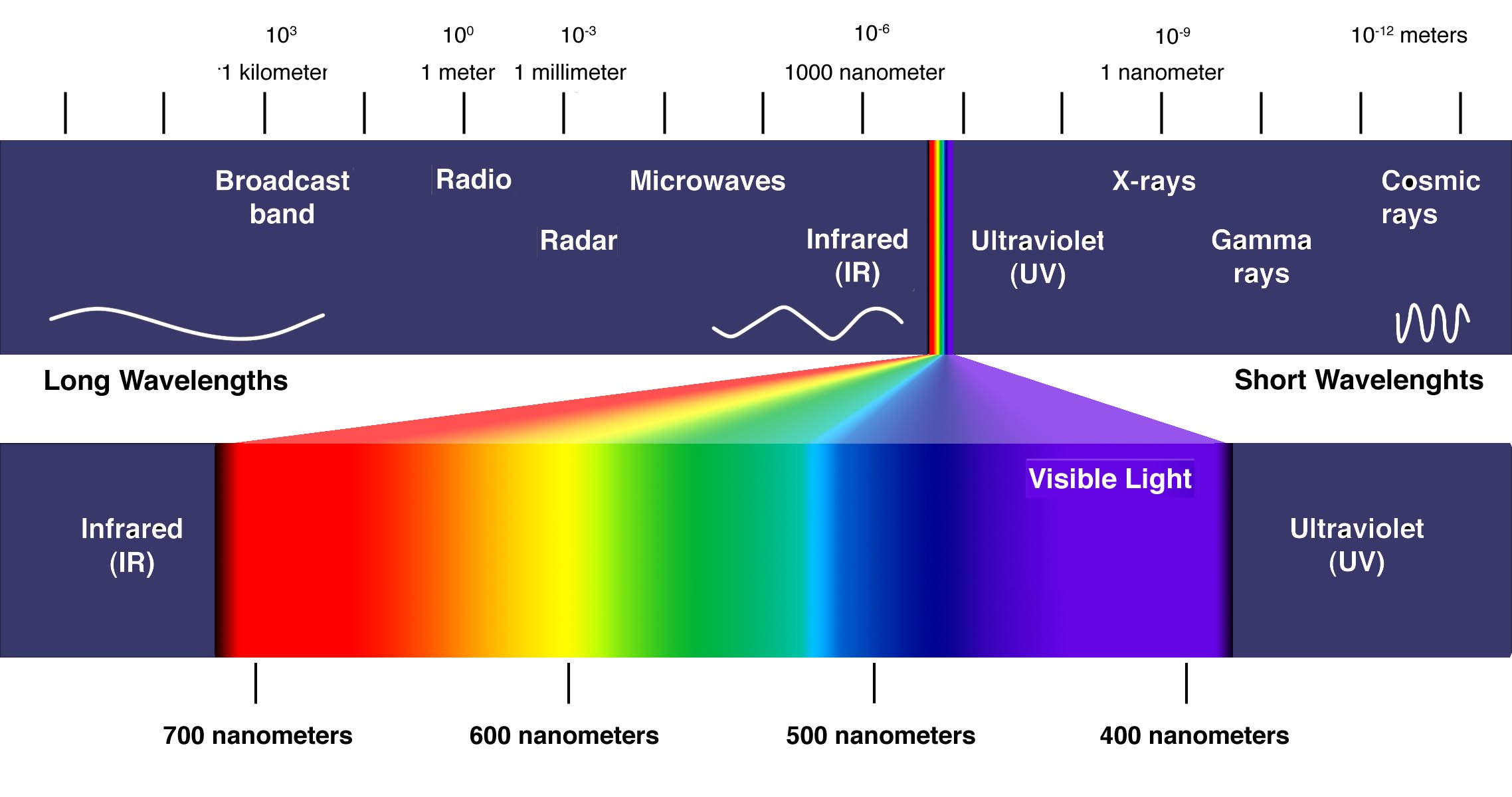
Emission Spectra Wavelength Range . Each of the absorption lines. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample.
from newgradoptometry.com
The actual wavelengths of the lines are predictably. Each of the absorption lines. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. When hydrogen gas is placed. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity.
Understanding Acuvue Contacts and Ultraviolet Light
Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The actual wavelengths of the lines are predictably. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Each of the absorption lines.
From www.researchgate.net
Fluorescence emission (blue and green), and excitation (red) spectra of Emission Spectra Wavelength Range In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. When hydrogen gas is placed. In chemistry, an emission spectrum refers to the range of wavelengths emitted by. Emission Spectra Wavelength Range.
From www.researchgate.net
The origins of three of the major sodium emission spectral lines (the Emission Spectra Wavelength Range In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The spectroscope separates the light of varying wavelengths. Emission Spectra Wavelength Range.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Wavelength Range In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In an emission spectrum, the excitation monochromator is set to. Emission Spectra Wavelength Range.
From www.heraeus.com
Emission spectra of infrared lamps Emission Spectra Wavelength Range When hydrogen gas is placed. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. The spectroscope separates the. Emission Spectra Wavelength Range.
From www.researchgate.net
Emission spectra of LED light sources. Download Scientific Diagram Emission Spectra Wavelength Range In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. Each of the absorption lines. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectrum (or line spectrum) of a chemical element. Emission Spectra Wavelength Range.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Wavelength Range The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Each of the absorption lines. The emission spectrum (or line spectrum) of a chemical. Emission Spectra Wavelength Range.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce Emission Spectra Wavelength Range The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The actual wavelengths of the lines are predictably. Each of the absorption lines. When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set. Emission Spectra Wavelength Range.
From www.animalia-life.club
Spectrum Wavelengths Chart Emission Spectra Wavelength Range When hydrogen gas is placed. The actual wavelengths of the lines are predictably. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The emission spectrum (or line. Emission Spectra Wavelength Range.
From www.researchgate.net
Fluorescence emission spectra (excitation wavelength = 365 nm) of the Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. In chemistry, an emission spectrum refers to the range of wavelengths. Emission Spectra Wavelength Range.
From analytik.co.uk
Visible Light Spectrum Analytik Ltd Emission Spectra Wavelength Range In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. When hydrogen gas is placed. Each of the absorption lines. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is. Emission Spectra Wavelength Range.
From www.youtube.com
Spectrum Basic Introduction YouTube Emission Spectra Wavelength Range When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The spectroscope separates the light of. Emission Spectra Wavelength Range.
From www.thoughtco.com
Visible Light Spectrum Overview and Chart Emission Spectra Wavelength Range The actual wavelengths of the lines are predictably. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes) is an analytical technique used to identify and. Emission Spectra Wavelength Range.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When hydrogen gas is placed. Each of the absorption lines. Atomic emission. Emission Spectra Wavelength Range.
From byjus.com
What do you mean by spectrum? Give the complete Emission Spectra Wavelength Range Each of the absorption lines. The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Atomic emission spectroscopy (aes). Emission Spectra Wavelength Range.
From www.researchgate.net
[(a)(c)] Typical emission spectra for the three wavelength ranges Emission Spectra Wavelength Range The actual wavelengths of the lines are predictably. When hydrogen gas is placed. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. In an emission spectrum, the excitation monochromator is set. Emission Spectra Wavelength Range.
From fphoto.photoshelter.com
science physics waves spectrum analysis Fundamental Emission Spectra Wavelength Range Each of the absorption lines. The actual wavelengths of the lines are predictably. When hydrogen gas is placed. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric. Emission Spectra Wavelength Range.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Spectra Wavelength Range Each of the absorption lines. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The emission spectrum. Emission Spectra Wavelength Range.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Each of the absorption lines. When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The. Emission Spectra Wavelength Range.
From atmos.washington.edu
Radiation spectrum for the Sun and Earth. Emission Spectra Wavelength Range In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In an emission spectrum, the excitation monochromator is set to. Emission Spectra Wavelength Range.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Wavelength Range Each of the absorption lines. The actual wavelengths of the lines are predictably. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat. Emission Spectra Wavelength Range.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. In chemistry, an emission spectrum refers to the range of. Emission Spectra Wavelength Range.
From www.faculty.luther.edu
Radiation Wavelength Emission Spectra Wavelength Range The actual wavelengths of the lines are predictably. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Each of the absorption lines. In an emission spectrum, the excitation monochromator. Emission Spectra Wavelength Range.
From stock.adobe.com
Continuous spectrum, an emission spectrum that consists of continuum of Emission Spectra Wavelength Range Each of the absorption lines. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. When hydrogen gas is placed. In chemistry, an emission spectrum refers to the. Emission Spectra Wavelength Range.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The actual wavelengths of the lines are predictably. The spectroscope separates. Emission Spectra Wavelength Range.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Emission Spectra Wavelength Range In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. Each of the absorption lines. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The spectroscope separates the light of varying wavelengths. Emission Spectra Wavelength Range.
From byjus.com
Emission Spectrum Atomic Spectra Comparison Chemistry Byju's Emission Spectra Wavelength Range The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. When hydrogen gas is. Emission Spectra Wavelength Range.
From www.researchgate.net
(a). Intensity of emission spectra versus wavelength for dry argon Emission Spectra Wavelength Range Each of the absorption lines. The actual wavelengths of the lines are predictably. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or electric current. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In an emission spectrum, the excitation monochromator is set. Emission Spectra Wavelength Range.
From telegra.ph
Understanding the Spectrum Telegraph Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The spectroscope separates the light of varying wavelengths and we observe. Emission Spectra Wavelength Range.
From www.elprocus.com
Vibgyor Colours Visible Spectrum, Wavelength and Frequency Emission Spectra Wavelength Range The spectroscope separates the light of varying wavelengths and we observe a line spectrum. Each of the absorption lines. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample. Emission Spectra Wavelength Range.
From www.alamy.com
Light spectrum color wavelength radiation prism line Emission Spectra Wavelength Range The actual wavelengths of the lines are predictably. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental. Emission Spectra Wavelength Range.
From armstrongeconomics.com
The Schema Frequency Armstrong Economics Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas is placed. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. Atomic emission spectroscopy (aes) is an. Emission Spectra Wavelength Range.
From www.researchgate.net
(a) Normalized emission, (b) actual emission, and (c) PLE spectra of Emission Spectra Wavelength Range The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The actual wavelengths of the lines are predictably. When hydrogen gas is placed. Each of the absorption lines. In chemistry,. Emission Spectra Wavelength Range.
From newgradoptometry.com
Understanding Acuvue Contacts and Ultraviolet Light Emission Spectra Wavelength Range The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The actual wavelengths of the lines are predictably. Each of the absorption lines. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator. Emission Spectra Wavelength Range.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectra Wavelength Range The actual wavelengths of the lines are predictably. In an emission spectrum, the excitation monochromator is set to some wavelength known to excite the sample and the emission monochromator is scanned. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. When hydrogen gas. Emission Spectra Wavelength Range.
From ozonedepletiontheory.info
What is Radiation Emission Spectra Wavelength Range Atomic emission spectroscopy (aes) is an analytical technique used to identify and quantify the elemental composition of a sample. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. When hydrogen gas is placed. In chemistry, an emission spectrum refers to the range of wavelengths emitted by an atom or compound stimulated by either heat or. Emission Spectra Wavelength Range.