Lead Unpaired Electrons . The shorthand electron configuration (or noble gas configuration) as well as. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; Lead is in the p block,. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. Electron configuration chart of all elements is mentioned in the table below. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Because all the 2 p orbitals are. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration.
from www.alamy.com
For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; Electron configuration chart of all elements is mentioned in the table below. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Lead is in the p block,. Because all the 2 p orbitals are. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. The shorthand electron configuration (or noble gas configuration) as well as.
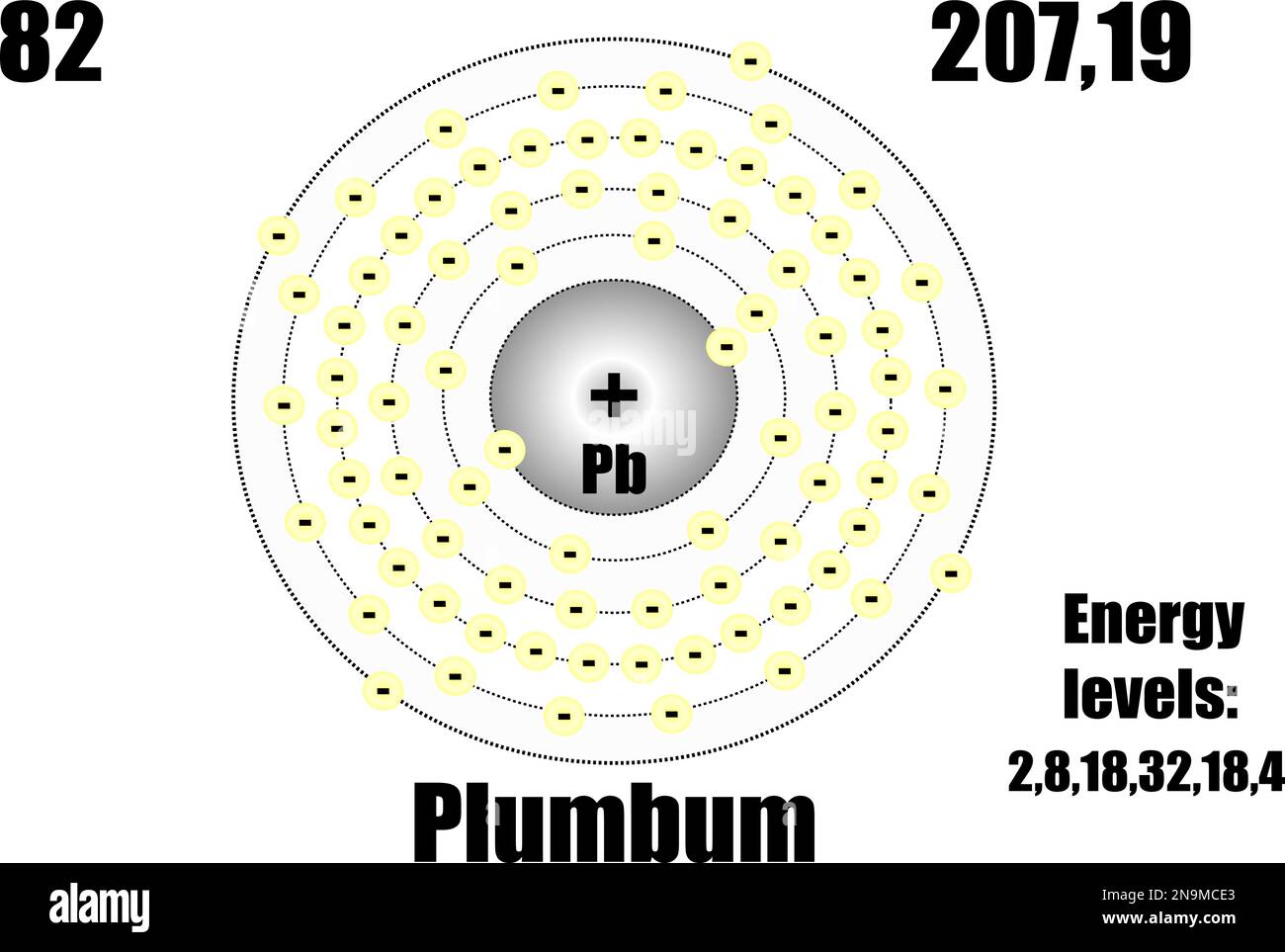
Lead atom, with mass and energy levels. Vector illustration Stock
Lead Unpaired Electrons One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration chart of all elements is mentioned in the table below. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; Lead is in the p block,. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. The shorthand electron configuration (or noble gas configuration) as well as. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Because all the 2 p orbitals are.
From www.slideserve.com
PPT Unit 7 Bonding & Molecular Geometry PowerPoint Presentation Lead Unpaired Electrons The shorthand electron configuration (or noble gas configuration) as well as. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Electron. Lead Unpaired Electrons.
From www.youtube.com
How to Find Unpaired Electrons of Coordination Compound YouTube Lead Unpaired Electrons Because all the 2 p orbitals are. Electron configuration chart of all elements is mentioned in the table below. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. This electron configuration calculator will instantly show you the distribution of electrons. Lead Unpaired Electrons.
From byjus.com
The number of unpaired electrons in carbon atom in excited state is Lead Unpaired Electrons Electron configuration chart of all elements is mentioned in the table below. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Lead is in the p. Lead Unpaired Electrons.
From byjus.com
Indicate the number of unpaired electrons in P. Lead Unpaired Electrons Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an. Lead Unpaired Electrons.
From byjus.com
How to find unpaired electron? Lead Unpaired Electrons Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Lead Unpaired Electrons.
From www.nagwa.com
Question Video Determining the Number of Unpaired Electrons in an Atom Lead Unpaired Electrons Because all the 2 p orbitals are. Lead is in the p block,. The shorthand electron configuration (or noble gas configuration) as well as. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. Electron configuration chart of all elements is. Lead Unpaired Electrons.
From manuallistcantabank.z21.web.core.windows.net
Electron Configuration Orbital Diagram Lead Unpaired Electrons This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins). Lead Unpaired Electrons.
From www.coursehero.com
[Solved] For each atom or ion, determine the number of unpaired Lead Unpaired Electrons Because all the 2 p orbitals are. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; In chemistry, an unpaired electron is an electron that occupies. Lead Unpaired Electrons.
From www.dreamstime.com
Lead stock illustration. Illustration of atomic, symbol 175796461 Lead Unpaired Electrons The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; Because all the 2 p orbitals are. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly,. Lead Unpaired Electrons.
From www.youtube.com
How To Determine The Number of Paired and Unpaired Electrons YouTube Lead Unpaired Electrons For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. Because all the 2 p orbitals are. Lead is in the p block,. In chemistry, an unpaired. Lead Unpaired Electrons.
From www.youtube.com
unpaired electrons & moment Atomic structure Lec.6 YouTube Lead Unpaired Electrons In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. For unpaired electrons, convention assigns the value. Lead Unpaired Electrons.
From mungfali.com
Unpaired Electrons Periodic Table Lead Unpaired Electrons Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. The shorthand electron configuration (or noble gas configuration) as well as. Because all the 2 p orbitals are. Electron configuration chart of all elements is mentioned in the table below. For. Lead Unpaired Electrons.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead Unpaired Electrons In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration chart of all elements is mentioned in the table below. Because all the 2 p. Lead Unpaired Electrons.
From courses.lumenlearning.com
3.4 Electronic Structure of Atoms (Electron Configurations) General Lead Unpaired Electrons Lead is in the p block,. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the. Lead Unpaired Electrons.
From byjus.com
The electronic configuration of a particular neutral atom is Lead Unpaired Electrons Electron configuration chart of all elements is mentioned in the table below. For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; The shorthand electron configuration (or noble gas configuration) as well as. Lead is in the p block,. One electron must be paired with another in one of the 2p orbitals, which gives us two. Lead Unpaired Electrons.
From sites.google.com
Unit 4 Lewis Dot Structures & Bonding Mrosla Science Lead Unpaired Electrons The shorthand electron configuration (or noble gas configuration) as well as. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Lead is in the p block,. Electron configuration chart of all elements is mentioned in the table below. Because all the 2 p orbitals are.. Lead Unpaired Electrons.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Unpaired Electrons Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Because all the 2 p orbitals are.. Lead Unpaired Electrons.
From www.researchgate.net
Evolution of unpaired electrons with molecular geometry. (a) Unpaired Lead Unpaired Electrons This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration chart of all elements is mentioned in the table below. Because all the 2 p orbitals are. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an. Lead Unpaired Electrons.
From www.alamy.com
Lead atom, with mass and energy levels. Vector illustration Stock Lead Unpaired Electrons For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; Because all the 2 p orbitals are. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Lead is in the p block,. Let's figure out the number of unpaired electrons in. Lead Unpaired Electrons.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead Unpaired Electrons This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Because all the. Lead Unpaired Electrons.
From www.toppr.com
The number of unpaired electrons in [NiCl4]^2 , Ni(CO)4 and [Cu(NH3)4 Lead Unpaired Electrons Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Lead is in the p block,. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. In chemistry, an unpaired electron is. Lead Unpaired Electrons.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes Lead Unpaired Electrons One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. This electron configuration calculator will instantly show. Lead Unpaired Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Unpaired Electrons The shorthand electron configuration (or noble gas configuration) as well as. Lead is in the p block,. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. Let's figure out the number of unpaired electrons in a lead atom first, before. Lead Unpaired Electrons.
From www.slideserve.com
PPT Chapter 10 Basic Concepts of Chemical Bonding PowerPoint Lead Unpaired Electrons Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. Lead is in the p block,. Because all the 2 p orbitals are. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic. Lead Unpaired Electrons.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Unpaired Electrons Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Lead is in the p block,. Electron configuration chart of all elements is mentioned in the table below. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The shorthand electron configuration. Lead Unpaired Electrons.
From cabinet.matttroy.net
Lead Periodic Table Electrons Matttroy Lead Unpaired Electrons Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Electron configuration chart of all elements is mentioned in the table below. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of.. Lead Unpaired Electrons.
From www.youtube.com
Number of unpaired electrons in Co+2 YouTube Lead Unpaired Electrons This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an. Lead Unpaired Electrons.
From www.slideserve.com
PPT Orbital Diagrams and Electron Configuration PowerPoint Lead Unpaired Electrons One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Let's figure out the number of unpaired. Lead Unpaired Electrons.
From www.youtube.com
Trick To Find Number Of Unpaired Electrons In 3d Metal Ions YouTube Lead Unpaired Electrons Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s. Lead Unpaired Electrons.
From www.researchgate.net
Dyson orbitals showing the unpaired electron in lowlying electronic Lead Unpaired Electrons One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Lead is in the p block,. Electron configuration chart of all elements. Lead Unpaired Electrons.
From byjus.com
The difference in the number of unpaired electrons in NiCl2 and [NiCN6 Lead Unpaired Electrons For unpaired electrons, convention assigns the value of \(+\dfrac{1}{2}\) for the spin quantum number; Electron configuration chart of all elements is mentioned in the table below. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Oxygen (atomic number 8) has a pair of electrons in any one of the 2. Lead Unpaired Electrons.
From chem.libretexts.org
8.5 Molecular Orbital Theory Chemistry LibreTexts Lead Unpaired Electrons One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. Lead is in the p block,. This electron configuration calculator will instantly. Lead Unpaired Electrons.
From www.coursehero.com
[Solved] For each atom or ion, determine the number of unpaired Lead Unpaired Electrons In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. One electron must be paired with another in one of the 2p orbitals, which gives us two. Lead Unpaired Electrons.
From byjus.com
10.The difference between number of shared electron pairs and unpaired Lead Unpaired Electrons Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the electrons have opposite spins) and a single electron in each of. Let's figure out the number of unpaired electrons in a lead atom first, before moving onto the lead ion. The shorthand electron configuration (or noble gas configuration) as well as. In. Lead Unpaired Electrons.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Lead Unpaired Electrons In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Because all the 2 p orbitals are. The shorthand electron configuration (or noble gas configuration) as well as. Oxygen (atomic number 8) has a pair of electrons in any one of the 2 p orbitals (the. Lead Unpaired Electrons.