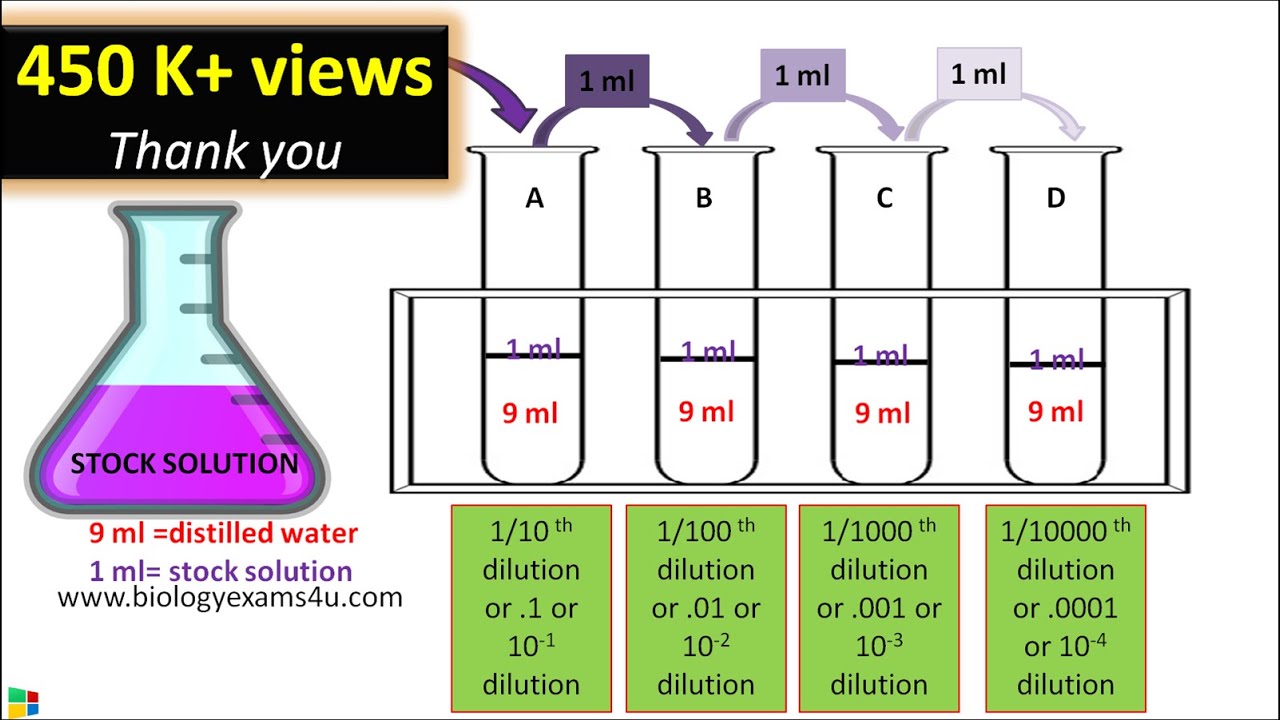
Dilution Lab Conclusion . Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Generate a standard curve and use the standard curve to determine the concentration of a solution. • use volumetric and mohr pipets and a. A dilute solution is one. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Preparing solutions and dilutions lab. We will begin our discussion of solution concentration with two related and relative terms: In both dilution and concentration, the. • prepare a dilute solution from a more concentrated one. Use the spectrophotometer to measure the absorbance of solutions.
from www.youtube.com
• prepare a dilute solution from a more concentrated one. Preparing solutions and dilutions lab. Use the spectrophotometer to measure the absorbance of solutions. A dilute solution is one. Generate a standard curve and use the standard curve to determine the concentration of a solution. In both dilution and concentration, the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. • use volumetric and mohr pipets and a. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory.
Serial Dilution Method Protocol Step Wise Explanation YouTube
Dilution Lab Conclusion Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. A dilute solution is one. Preparing solutions and dilutions lab. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Generate a standard curve and use the standard curve to determine the concentration of a solution. We will begin our discussion of solution concentration with two related and relative terms: • use volumetric and mohr pipets and a. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration, the. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. • prepare a dilute solution from a more concentrated one. Use the spectrophotometer to measure the absorbance of solutions.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Lab Conclusion Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. • prepare a dilute solution from a more concentrated one. In both dilution and concentration, the. Generate a standard curve and use the standard curve to determine the concentration of a solution. Preparing solutions and dilutions lab. We will begin our discussion of solution concentration. Dilution Lab Conclusion.
From fasrhealthcare850.weebly.com
Serial Dilution Lab Report Microbiology fasrhealthcare Dilution Lab Conclusion Generate a standard curve and use the standard curve to determine the concentration of a solution. Use the spectrophotometer to measure the absorbance of solutions. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. • use volumetric. Dilution Lab Conclusion.
From czcasini.weebly.com
serial dilution lab conclusion czcasini Dilution Lab Conclusion Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. A dilute solution is one. Preparing solutions and dilutions lab. • prepare a dilute solution from a more concentrated one. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. In both dilution and concentration, the. Generate a. Dilution Lab Conclusion.
From australianenergy.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion Use the spectrophotometer to measure the absorbance of solutions. We will begin our discussion of solution concentration with two related and relative terms: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. In both dilution and concentration, the. Two skills that are essential to a chemist are preparing. Dilution Lab Conclusion.
From www.scribd.com
Lab Report Dilution Molar Concentration Solution Dilution Lab Conclusion Generate a standard curve and use the standard curve to determine the concentration of a solution. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Use the spectrophotometer to measure the absorbance of solutions. A dilute solution is one. We will begin our discussion of solution concentration with two related and relative. Dilution Lab Conclusion.
From energystaffing.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion In both dilution and concentration, the. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. • use volumetric and mohr pipets and a. Use the spectrophotometer to measure the absorbance of solutions. • prepare a dilute solution from a more concentrated one. Mastering the art of dilutions is a crucial skill for. Dilution Lab Conclusion.
From buildersos.weebly.com
Serial dilution lab conclusion buildersos Dilution Lab Conclusion A dilute solution is one. We will begin our discussion of solution concentration with two related and relative terms: Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Use the spectrophotometer to measure the absorbance of solutions. • prepare a dilute solution from a more concentrated one. In both dilution and concentration, the. •. Dilution Lab Conclusion.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Lab Conclusion Preparing solutions and dilutions lab. We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Use the spectrophotometer to measure the absorbance of solutions. Dilution is the addition of solvent, which decreases the concentration of the. Dilution Lab Conclusion.
From massivepolaris.tistory.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion Use the spectrophotometer to measure the absorbance of solutions. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Generate a standard curve and use the standard curve to determine the concentration of a solution. A dilute solution is one. Preparing solutions and dilutions lab. Dilution is the addition of solvent, which decreases. Dilution Lab Conclusion.
From www.scribd.com
78 Dilution Lab Experiment Solution Free 30day Trial Scribd Dilution Lab Conclusion In both dilution and concentration, the. Generate a standard curve and use the standard curve to determine the concentration of a solution. • prepare a dilute solution from a more concentrated one. Preparing solutions and dilutions lab. We will begin our discussion of solution concentration with two related and relative terms: Mastering the art of dilutions is a crucial skill. Dilution Lab Conclusion.
From zoneenergy.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion In both dilution and concentration, the. We will begin our discussion of solution concentration with two related and relative terms: • use volumetric and mohr pipets and a. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Generate a standard curve and use the standard curve to determine the concentration of a. Dilution Lab Conclusion.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Lab Conclusion Generate a standard curve and use the standard curve to determine the concentration of a solution. Use the spectrophotometer to measure the absorbance of solutions. • prepare a dilute solution from a more concentrated one. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Dilution is the addition of solvent, which decreases the concentration. Dilution Lab Conclusion.
From www.slideserve.com
PPT May 24 th 1 st Get Lab Notebooks, Get into groups PowerPoint Dilution Lab Conclusion Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Generate a standard curve and use the standard curve to determine the concentration of a solution. • use volumetric and mohr pipets and a. Preparing solutions and dilutions lab. We will begin our discussion of solution concentration with two related and relative terms: Use. Dilution Lab Conclusion.
From bestlinebasket.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. A dilute solution is one. • use volumetric and mohr pipets and a. In both dilution and concentration, the. Generate a standard curve and use the standard curve to determine the concentration of a solution. • prepare a dilute solution from a more. Dilution Lab Conclusion.
From studylib.net
Student Lab Serial Dilution and Chemical Toxicity Dilution Lab Conclusion Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. In both dilution and concentration, the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. • use volumetric and mohr pipets and a. Mastering the art of dilutions is a crucial skill for any scientist in. Dilution Lab Conclusion.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Lab Conclusion We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. • prepare a dilute solution from a more concentrated one. In both dilution and concentration, the. Dilution is the addition of solvent, which decreases. Dilution Lab Conclusion.
From mojolimfa.weebly.com
Serial dilution lab conclusion mojolimfa Dilution Lab Conclusion We will begin our discussion of solution concentration with two related and relative terms: A dilute solution is one. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Use the spectrophotometer to measure the absorbance of solutions. Generate a standard curve and use the standard curve to determine the concentration of a. Dilution Lab Conclusion.
From apdelali.weebly.com
Serial Dilution Lab Conclusion jenyamil Dilution Lab Conclusion A dilute solution is one. Preparing solutions and dilutions lab. Generate a standard curve and use the standard curve to determine the concentration of a solution. Use the spectrophotometer to measure the absorbance of solutions. In both dilution and concentration, the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. • use volumetric. Dilution Lab Conclusion.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Dilution Lab Conclusion Generate a standard curve and use the standard curve to determine the concentration of a solution. In both dilution and concentration, the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. • use volumetric and mohr pipets and a. A dilute solution is one. We will begin our discussion of solution concentration with. Dilution Lab Conclusion.
From studylib.net
Well, What Will We Drink? Ppm, ppb, and Serial Dilution Lab Dilution Lab Conclusion • prepare a dilute solution from a more concentrated one. Generate a standard curve and use the standard curve to determine the concentration of a solution. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. In both dilution and concentration, the. Two skills that are essential to a chemist are preparing solutions of known. Dilution Lab Conclusion.
From www.studocu.com
16 Dilution 13 hmmm Experiment 16 The Solution is Dilution Dilution Lab Conclusion Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. A dilute solution is one. Generate a standard curve and use the standard curve to determine the concentration of a solution. In both dilution and concentration, the. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. •. Dilution Lab Conclusion.
From ecseoseosk.netlify.app
Serial Dilution Lab Conclusion Dilution Lab Conclusion We will begin our discussion of solution concentration with two related and relative terms: Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. • prepare a dilute solution from a more concentrated one. • use volumetric and mohr pipets and a. In both dilution and concentration, the. Generate a standard curve and. Dilution Lab Conclusion.
From swgoodtext.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. • prepare a dilute solution from a more concentrated one. In both dilution and concentration, the. Preparing solutions and dilutions lab. Use the spectrophotometer to measure the absorbance. Dilution Lab Conclusion.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Lab Conclusion Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Preparing solutions and dilutions lab. Generate a standard curve and use the standard curve to determine the concentration of a solution. We. Dilution Lab Conclusion.
From klaunwhgn.blob.core.windows.net
How To Do Dilutions at Margaret Krueger blog Dilution Lab Conclusion A dilute solution is one. • use volumetric and mohr pipets and a. Preparing solutions and dilutions lab. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We will begin our discussion of solution concentration with two related and relative terms: Generate a standard curve and use the standard curve to determine the. Dilution Lab Conclusion.
From www.studocu.com
Dilutions Lab Two . Dilutions For many years, scientists have used Dilution Lab Conclusion In both dilution and concentration, the. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. We will begin our discussion of. Dilution Lab Conclusion.
From www.studocu.com
Serial Dilutions Lab Report Making Serial Dilutions INTRODUCTION Dilution Lab Conclusion Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. In both dilution and concentration, the. • prepare a dilute solution from a more concentrated one. Preparing solutions and dilutions lab. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. • use volumetric and mohr pipets and. Dilution Lab Conclusion.
From allworldre.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. A dilute solution is one. • prepare a dilute solution from a more concentrated one. Generate a standard curve and use the standard curve to determine the concentration of a solution. In both dilution and concentration, the. Preparing solutions and dilutions lab. Two skills that. Dilution Lab Conclusion.
From chemistryknights.blogspot.com
Chemistry Knights VIDEO DILUTION LAB CALCULATIONS Dilution Lab Conclusion We will begin our discussion of solution concentration with two related and relative terms: Use the spectrophotometer to measure the absorbance of solutions. A dilute solution is one. • prepare a dilute solution from a more concentrated one. Generate a standard curve and use the standard curve to determine the concentration of a solution. Dilution is the addition of solvent,. Dilution Lab Conclusion.
From kenpulse.weebly.com
Serial Dilution Lab Conclusion kenpulse Dilution Lab Conclusion • prepare a dilute solution from a more concentrated one. In both dilution and concentration, the. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Preparing solutions and dilutions lab. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. Use the spectrophotometer to measure the absorbance. Dilution Lab Conclusion.
From studylib.net
Concentration and Dilution Lab Dilution Lab Conclusion A dilute solution is one. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. In both dilution and concentration, the. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Generate a standard curve and use the standard curve to determine the concentration of a solution. • prepare. Dilution Lab Conclusion.
From bedmegabest.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion Use the spectrophotometer to measure the absorbance of solutions. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. • prepare a dilute solution from a more concentrated one. Preparing solutions and dilutions lab. In both dilution and concentration,. Dilution Lab Conclusion.
From www.youtube.com
Dilution Lab Results Chemistry Matters YouTube Dilution Lab Conclusion Generate a standard curve and use the standard curve to determine the concentration of a solution. Two skills that are essential to a chemist are preparing solutions of known molarity (m) and diluting. A dilute solution is one. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. We will begin our discussion of solution. Dilution Lab Conclusion.
From nolasunion.weebly.com
serial dilution lab conclusion experiment nolasunion Dilution Lab Conclusion A dilute solution is one. In both dilution and concentration, the. Generate a standard curve and use the standard curve to determine the concentration of a solution. Mastering the art of dilutions is a crucial skill for any scientist in the laboratory. We will begin our discussion of solution concentration with two related and relative terms: Preparing solutions and dilutions. Dilution Lab Conclusion.
From everythingband.web.fc2.com
Serial Dilution Lab Conclusion Dilution Lab Conclusion Use the spectrophotometer to measure the absorbance of solutions. Generate a standard curve and use the standard curve to determine the concentration of a solution. Preparing solutions and dilutions lab. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. • prepare a dilute solution from a more concentrated one. In both dilution and. Dilution Lab Conclusion.