Medical Device Database Singapore . Licensed importers, wholesalers or manufacturers of health. Class a medical device database. Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. View medical device information online and carry out transactions with our medical device branch. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. Registering medical devices in singapore classification and regulatory pathways. The health sciences authority (hsa). The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). You are encouraged to check if your product is considered a medical device in singapore. You will need to determine your medical device’s risk.
from www.proximacro.com
You are encouraged to check if your product is considered a medical device in singapore. The health sciences authority (hsa). Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. Licensed importers, wholesalers or manufacturers of health. Class a medical device database. You will need to determine your medical device’s risk. Registering medical devices in singapore classification and regulatory pathways. View medical device information online and carry out transactions with our medical device branch.
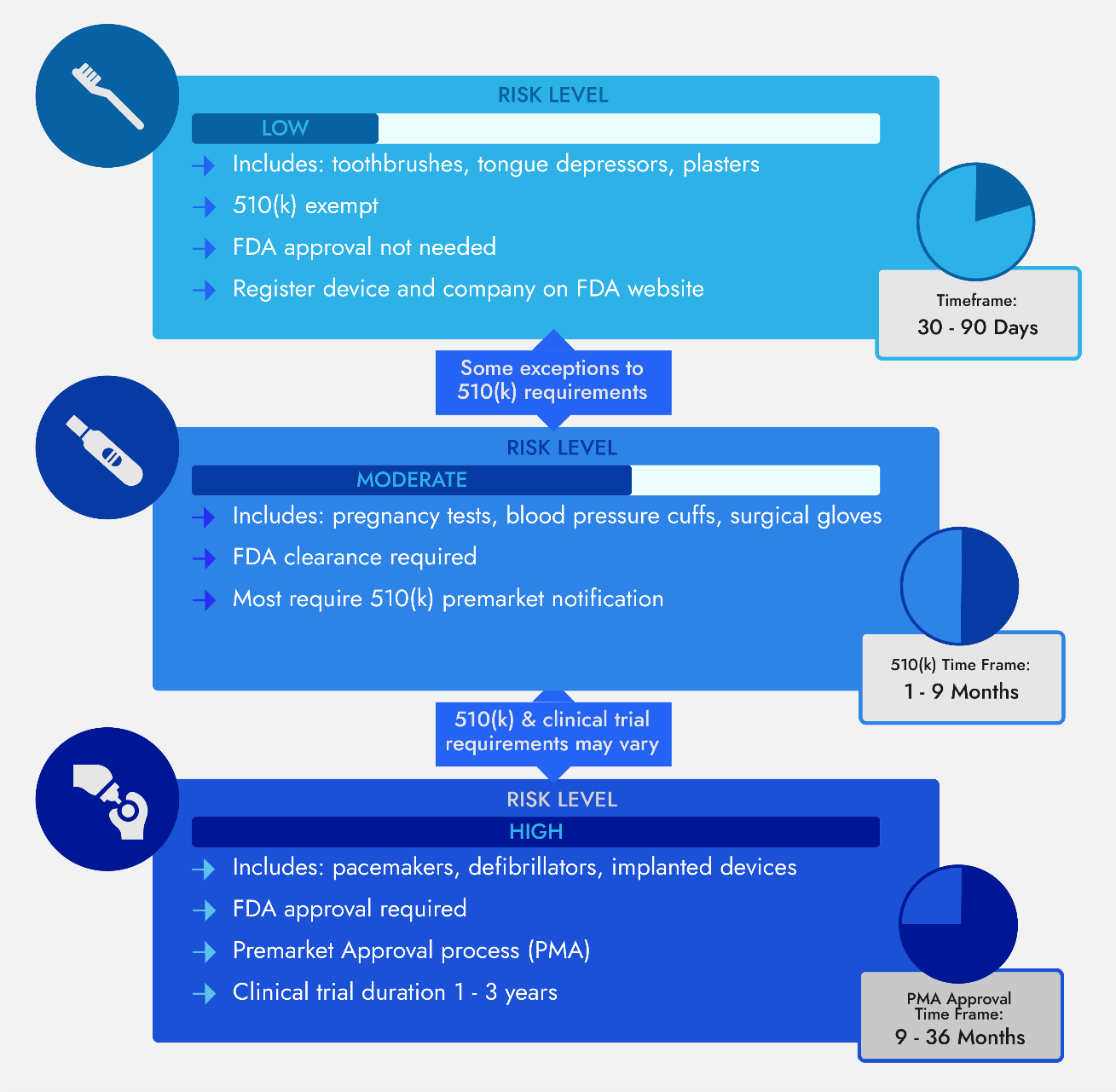
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA
Medical Device Database Singapore The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). Registering medical devices in singapore classification and regulatory pathways. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. View medical device information online and carry out transactions with our medical device branch. You are encouraged to check if your product is considered a medical device in singapore. You will need to determine your medical device’s risk. The health sciences authority (hsa). Class a medical device database. Licensed importers, wholesalers or manufacturers of health. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access.
From simplewebguide.com
Singapore Regulations on Healthcare Products Medical Device Database Singapore Registering medical devices in singapore classification and regulatory pathways. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). You will need to determine your medical device’s risk. Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. Class a medical device database. This search enables you to get. Medical Device Database Singapore.
From timly.com
Medical Device Management in Healthcare Sector Made Easy Medical Device Database Singapore Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. You are encouraged to check if your product is considered a medical device in singapore. Licensed importers, wholesalers or manufacturers of health. Registering medical devices in singapore classification and regulatory pathways. The health sciences authority (hsa). We can help medical device manufacturers comply with regulatory approval standards in. Medical Device Database Singapore.
From cmsmedtech.com
Medical Device registration in Singapore, Health Sciences Authority Medical Device Database Singapore The health sciences authority (hsa). Licensed importers, wholesalers or manufacturers of health. Registering medical devices in singapore classification and regulatory pathways. Class a medical device database. You will need to determine your medical device’s risk. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. This search enables. Medical Device Database Singapore.
From www.freyrsolutions.com
European Database on Medical Devices Freyr Global Regulatory Medical Device Database Singapore We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. View medical device information online and carry out transactions with our medical device branch. The health sciences authority. Medical Device Database Singapore.
From www.youtube.com
Medical Device Regulatory in Asia_Singapore YouTube Medical Device Database Singapore Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. Licensed importers, wholesalers or manufacturers of health. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). The health sciences authority (hsa). You will need to determine your medical device’s risk. You are encouraged to check if your product. Medical Device Database Singapore.
From www.youtube.com
The Singapore Regulatory and Registration Procedures for the Sale of Medical Device Database Singapore Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. View medical device information online and carry out transactions with our medical device branch. Registering medical devices in singapore classification and regulatory pathways. The health sciences authority (hsa). Licensed importers, wholesalers or manufacturers of health. You will need to determine your medical device’s risk. We can help medical. Medical Device Database Singapore.
From www.pacificbridgemedical.com
Classification of General Medical Devices in Singapore Medical Device Database Singapore Class a medical device database. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. Registering medical devices in singapore classification and regulatory pathways. The health sciences authority (hsa). The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). You will. Medical Device Database Singapore.
From www.argosmultilingual.com
Medical Device Database Argos Multilingual Medical Device Database Singapore The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. Registering medical devices in singapore classification and regulatory pathways. The health sciences authority (hsa). Licensed importers, wholesalers or manufacturers of health. Class a medical device database. This search enables. Medical Device Database Singapore.
From www.regdesk.co
HSA Guidance on Medical Device Product Registration Class C and D Medical Device Database Singapore We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. Class a medical device database. Registering medical devices in singapore classification and regulatory pathways. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). Find out if your medicine/medical. Medical Device Database Singapore.
From www.regdesk.co
HSA Guidance on Grouping of Medical Devices Overview RegDesk Medical Device Database Singapore You will need to determine your medical device’s risk. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. You are encouraged to check if your product is considered a medical device in singapore. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique. Medical Device Database Singapore.
From www.slideshare.net
6 medical device registration in singapore Medical Device Database Singapore You will need to determine your medical device’s risk. Registering medical devices in singapore classification and regulatory pathways. View medical device information online and carry out transactions with our medical device branch. The health sciences authority (hsa). This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. We can help. Medical Device Database Singapore.
From www.youtube.com
How to use the International Medical Device Database Implant FIles Medical Device Database Singapore View medical device information online and carry out transactions with our medical device branch. Licensed importers, wholesalers or manufacturers of health. You will need to determine your medical device’s risk. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. We can help medical device manufacturers comply with regulatory approval. Medical Device Database Singapore.
From studylib.net
Universal Medical Device Nomenclature System WHO Informal Consultation Medical Device Database Singapore Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. The health sciences authority (hsa). Registering medical devices in singapore classification and regulatory pathways. Class a medical device database. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. You will need to determine your medical. Medical Device Database Singapore.
From operonstrategist.com
Wearable Medical Device Registration in Singapore (StepbyStep Medical Device Database Singapore View medical device information online and carry out transactions with our medical device branch. You will need to determine your medical device’s risk. You are encouraged to check if your product is considered a medical device in singapore. Registering medical devices in singapore classification and regulatory pathways. The health sciences authority (hsa). Licensed importers, wholesalers or manufacturers of health. We. Medical Device Database Singapore.
From www.regdesk.co
HSA Guidance on Medical Device Product Registration Additional Aspects Medical Device Database Singapore We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. Registering medical devices in singapore classification and regulatory pathways. Class a medical device database. You will need to determine your medical device’s risk. Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. The health sciences. Medical Device Database Singapore.
From credevo.com
Medical Device Registration Process in Singapore Credevo Articles Medical Device Database Singapore Class a medical device database. Licensed importers, wholesalers or manufacturers of health. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. View medical device information online and carry out transactions with our medical device branch. You are encouraged to check if your product is considered a medical device in. Medical Device Database Singapore.
From cmsmedtech.com
Medical Device registration in Singapore, Health Sciences Authority Medical Device Database Singapore View medical device information online and carry out transactions with our medical device branch. Registering medical devices in singapore classification and regulatory pathways. You will need to determine your medical device’s risk. The health sciences authority (hsa). We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. Class. Medical Device Database Singapore.
From www.syscreations.com
Medical Device Integration Solutions With EMR, EHR, LIS, HIS Medical Device Database Singapore Class a medical device database. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). You are encouraged to check if your product is considered a medical device in singapore. Licensed importers, wholesalers or manufacturers of health. We can help medical device manufacturers comply with regulatory approval standards in singapore under. Medical Device Database Singapore.
From saisystems.com
Improving with Medical Data Processing Saisystems Health Medical Device Database Singapore Licensed importers, wholesalers or manufacturers of health. You will need to determine your medical device’s risk. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. Class a medical device database. Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. Registering medical devices in singapore classification and. Medical Device Database Singapore.
From cmsmedtech.com
Medical Device registration in Singapore, Health Sciences Authority Medical Device Database Singapore Registering medical devices in singapore classification and regulatory pathways. View medical device information online and carry out transactions with our medical device branch. Licensed importers, wholesalers or manufacturers of health. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). You are encouraged to check if your product is considered a. Medical Device Database Singapore.
From desertplatforms.com
FDA Medical Device Databases Desert Platforms Medical Device Database Singapore This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. View medical device information online and carry out transactions with our medical device branch. Class a medical device database. You will need to determine your medical device’s risk. We can help medical device manufacturers comply with regulatory approval standards in. Medical Device Database Singapore.
From www.proximacro.com
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA Medical Device Database Singapore Licensed importers, wholesalers or manufacturers of health. Registering medical devices in singapore classification and regulatory pathways. View medical device information online and carry out transactions with our medical device branch. Class a medical device database. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. You are encouraged to check. Medical Device Database Singapore.
From globalregulatorypartners.com
Singapore's HSA Global Regulatory Partners, Inc. Medical Device Database Singapore The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. Registering medical devices in singapore classification and regulatory pathways.. Medical Device Database Singapore.
From emmainternational.com
FDA Medical Device Databases Backend Platforms Medical Device Database Singapore Class a medical device database. Licensed importers, wholesalers or manufacturers of health. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. The health sciences authority (hsa). You will need to determine your medical device’s risk. You are encouraged to check if your product is considered a medical device in. Medical Device Database Singapore.
From www.thompsoncoburn.com
FDA launches medical device database AccessGUDID Medical Device Database Singapore Licensed importers, wholesalers or manufacturers of health. Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. Class a medical device database. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. Registering medical devices in singapore classification and regulatory pathways. The purpose of this guidance. Medical Device Database Singapore.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Medical Device Database Singapore Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. Licensed importers, wholesalers or manufacturers of health. View medical device information online and carry out transactions with our medical device branch. Registering medical devices in singapore classification and regulatory pathways. Class a medical device database. The purpose of this guidance document is to provide clarity on the regulatory. Medical Device Database Singapore.
From desertplatforms.com
FDA Medical Device Databases Desert Platforms Medical Device Database Singapore The health sciences authority (hsa). Find out if your medicine/medical devices/chinese proprietary medicines are registered/listed in singapore. Licensed importers, wholesalers or manufacturers of health. View medical device information online and carry out transactions with our medical device branch. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). You will need. Medical Device Database Singapore.
From researchforecast.com
Singapore Medical Devices Market Analysis, Revenue, Major Players, Size Medical Device Database Singapore You are encouraged to check if your product is considered a medical device in singapore. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). Find out if your medicine/medical devices/chinese. Medical Device Database Singapore.
From cmsmedtech.com
Medical Device registration in Singapore, Health Sciences Authority Medical Device Database Singapore You will need to determine your medical device’s risk. You are encouraged to check if your product is considered a medical device in singapore. Licensed importers, wholesalers or manufacturers of health. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). Registering medical devices in singapore classification and regulatory pathways. We. Medical Device Database Singapore.
From medicaldevices.icij.org
International Medical Devices Database Medical Device Database Singapore You will need to determine your medical device’s risk. Registering medical devices in singapore classification and regulatory pathways. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. You are encouraged to check if your product is considered a medical device in singapore. Find out if your medicine/medical. Medical Device Database Singapore.
From www.pacificbridgemedical.com
Medical Device Submission Process in Singapore Infographic Medical Device Database Singapore The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). View medical device information online and carry out transactions with our medical device branch. You are encouraged to check if your product is considered a medical device in singapore. Registering medical devices in singapore classification and regulatory pathways. Class a medical. Medical Device Database Singapore.
From www.regdesk.co
HSA Guidance on Medical Device Registration Basics RegDesk Medical Device Database Singapore You are encouraged to check if your product is considered a medical device in singapore. Class a medical device database. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. The health sciences authority (hsa). This search enables you to get a listing of all registered therapeutic products. Medical Device Database Singapore.
From veranex.com
Challenges for Medical Device Database Builds and Strategies to Medical Device Database Singapore The health sciences authority (hsa). We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. View medical device information online and carry out transactions with our medical device branch. You are encouraged to check if your product is considered a medical device in singapore. The purpose of this. Medical Device Database Singapore.
From www.socotec-certification-international.sg
SS 6202016 (2021) Good Distribution Practice for Medical Devices Medical Device Database Singapore You will need to determine your medical device’s risk. This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. The purpose of this guidance document is to provide clarity on the regulatory requirements for unique device identification (udi). The health sciences authority (hsa). You are encouraged to check if your. Medical Device Database Singapore.
From www.linkedin.com
The Singapore Guidance on Software Medical Devices Medical Device Database Singapore This search enables you to get a listing of all registered therapeutic products in singapore and their current approved package. Registering medical devices in singapore classification and regulatory pathways. Licensed importers, wholesalers or manufacturers of health. We can help medical device manufacturers comply with regulatory approval standards in singapore under the health sciences authority (hsa) for market access. Find out. Medical Device Database Singapore.