Titration Curve Ap Chemistry . The following titration curve shows the change in. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. What i absolutely have to know to survive the ap exam. There are four main points in a titration curve: The region where the titrant is added. The start where the solution only contains either acid or base. Effortlessly generate detailed titration curves for strong acid and strong base reactions. (e) use the titration curve and the information above to (i). The following might indicate the question deals with buffers and/or titrations: Ph of the solution during the titration. Choosing the best indicator for different titrations depending on the ph at the equivalence.
from general.chemistrysteps.com
What i absolutely have to know to survive the ap exam. Effortlessly generate detailed titration curves for strong acid and strong base reactions. The start where the solution only contains either acid or base. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Choosing the best indicator for different titrations depending on the ph at the equivalence. (e) use the titration curve and the information above to (i). There are four main points in a titration curve: Ph of the solution during the titration. The following might indicate the question deals with buffers and/or titrations: The following titration curve shows the change in.
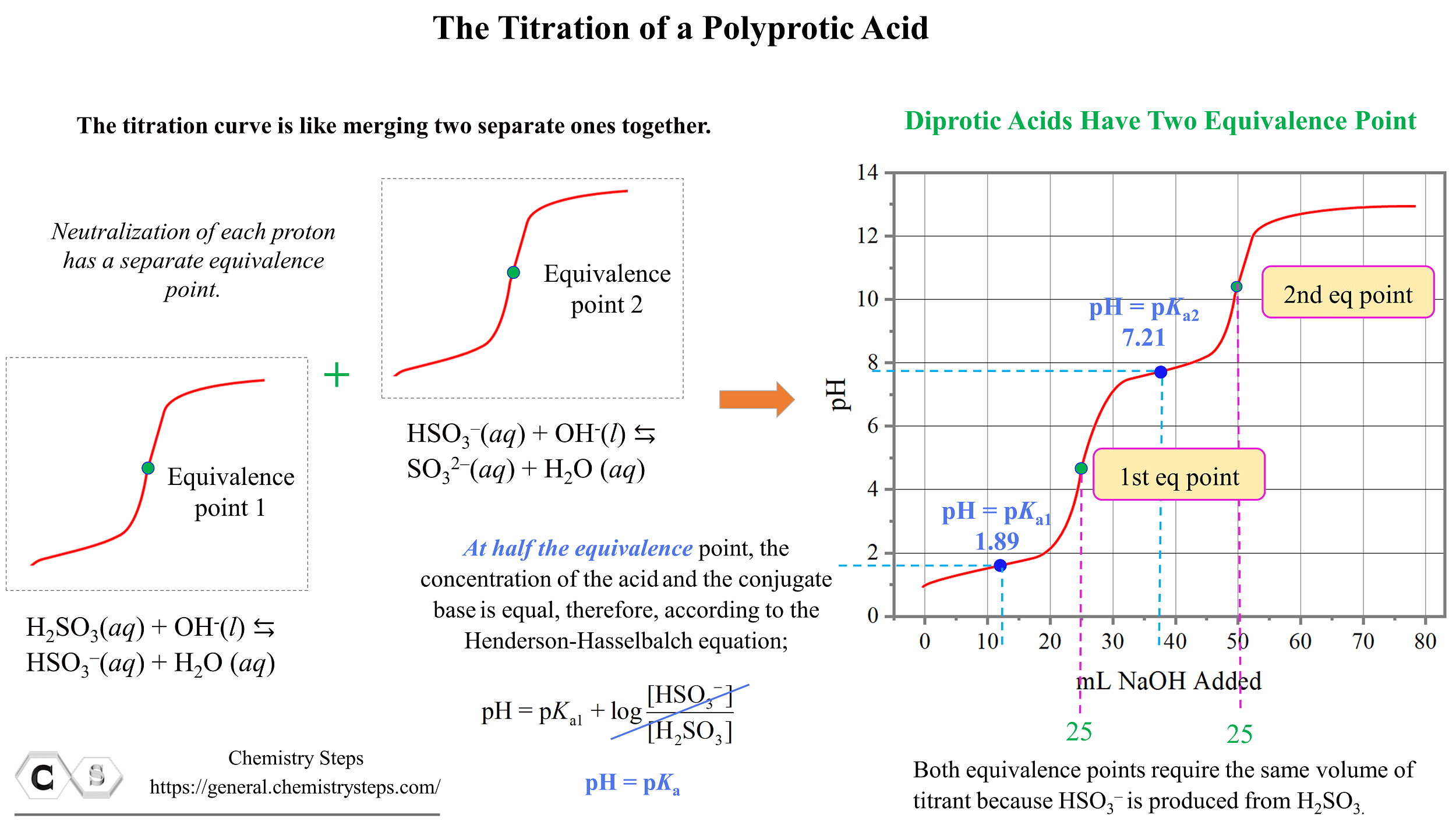
Titration of a Polyprotic Acids Chemistry Steps
Titration Curve Ap Chemistry Choosing the best indicator for different titrations depending on the ph at the equivalence. What i absolutely have to know to survive the ap exam. Choosing the best indicator for different titrations depending on the ph at the equivalence. (e) use the titration curve and the information above to (i). Ph of the solution during the titration. The start where the solution only contains either acid or base. The region where the titrant is added. There are four main points in a titration curve: The following might indicate the question deals with buffers and/or titrations: Effortlessly generate detailed titration curves for strong acid and strong base reactions. The following titration curve shows the change in. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: There are four main points in a titration curve: Effortlessly generate detailed titration curves for strong acid and strong base reactions. Ph of the solution during the titration. The following titration curve shows the change in. What i absolutely have to know to survive the ap exam. The region. Titration Curve Ap Chemistry.
From www.youtube.com
AP Chem Titration Part 2 YouTube Titration Curve Ap Chemistry The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. (e) use the titration curve and the information above to (i). Effortlessly generate detailed titration curves for strong acid and strong base reactions. The start where the solution only contains either acid or base.. Titration Curve Ap Chemistry.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Curve Ap Chemistry There are four main points in a titration curve: The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. (e) use the titration curve and the information above to (i). The region where the titrant is added. Effortlessly generate detailed titration curves for strong. Titration Curve Ap Chemistry.
From www.youtube.com
TRU Chemistry labs How To Plot a Titration Curve YouTube Titration Curve Ap Chemistry The region where the titrant is added. The following titration curve shows the change in. There are four main points in a titration curve: The following might indicate the question deals with buffers and/or titrations: The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the. Titration Curve Ap Chemistry.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve Ap Chemistry (e) use the titration curve and the information above to (i). What i absolutely have to know to survive the ap exam. Choosing the best indicator for different titrations depending on the ph at the equivalence. The following titration curve shows the change in. The start where the solution only contains either acid or base. There are four main points. Titration Curve Ap Chemistry.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Ap Chemistry The start where the solution only contains either acid or base. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Choosing the best indicator for different titrations depending on the ph at the equivalence. The following might indicate the question deals with buffers. Titration Curve Ap Chemistry.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Ap Chemistry Choosing the best indicator for different titrations depending on the ph at the equivalence. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Effortlessly generate detailed titration curves for strong acid and strong base reactions. There are four main points in a titration. Titration Curve Ap Chemistry.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curve Ap Chemistry Ph of the solution during the titration. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Effortlessly generate detailed titration curves for strong acid and strong base reactions. There are four main points in a titration curve: Choosing the best indicator for different. Titration Curve Ap Chemistry.
From www.youtube.com
AP Chem unit 8.5 Titration Curves YouTube Titration Curve Ap Chemistry The start where the solution only contains either acid or base. The following titration curve shows the change in. There are four main points in a titration curve: Choosing the best indicator for different titrations depending on the ph at the equivalence. Ph of the solution during the titration. The following might indicate the question deals with buffers and/or titrations:. Titration Curve Ap Chemistry.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curve Ap Chemistry The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. There are four main points in a titration curve: The following titration curve shows the change in. Ph of the solution during the titration. What i absolutely have to know to survive the ap. Titration Curve Ap Chemistry.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Titration Curve Ap Chemistry There are four main points in a titration curve: (e) use the titration curve and the information above to (i). The region where the titrant is added. Choosing the best indicator for different titrations depending on the ph at the equivalence. The following might indicate the question deals with buffers and/or titrations: Ph of the solution during the titration. The. Titration Curve Ap Chemistry.
From www.studocu.com
15 Demystifying Titration Curves AP* Chemistry Demystifying Titration Titration Curve Ap Chemistry The start where the solution only contains either acid or base. Choosing the best indicator for different titrations depending on the ph at the equivalence. The following titration curve shows the change in. The following might indicate the question deals with buffers and/or titrations: What i absolutely have to know to survive the ap exam. The amount of added strong. Titration Curve Ap Chemistry.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: (e) use the titration curve and the information above to (i). The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. The region where the titrant is added. There are four main points. Titration Curve Ap Chemistry.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: The start where the solution only contains either acid or base. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Choosing the best indicator for different titrations depending on the ph at. Titration Curve Ap Chemistry.
From www.youtube.com
AP Chemistry Titration Curves YouTube Titration Curve Ap Chemistry The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. The start where the solution only contains either acid or base. What i absolutely have to know to survive the ap exam. The following titration curve shows the change in. The region where the. Titration Curve Ap Chemistry.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Ap Chemistry The start where the solution only contains either acid or base. Ph of the solution during the titration. Choosing the best indicator for different titrations depending on the ph at the equivalence. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. The region. Titration Curve Ap Chemistry.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Ap Chemistry Effortlessly generate detailed titration curves for strong acid and strong base reactions. The following might indicate the question deals with buffers and/or titrations: Choosing the best indicator for different titrations depending on the ph at the equivalence. Ph of the solution during the titration. (e) use the titration curve and the information above to (i). The following titration curve shows. Titration Curve Ap Chemistry.
From www.youtube.com
AP Chemistry Notes 6.10 (Titration Curves) YouTube Titration Curve Ap Chemistry Choosing the best indicator for different titrations depending on the ph at the equivalence. The following might indicate the question deals with buffers and/or titrations: Ph of the solution during the titration. The start where the solution only contains either acid or base. Effortlessly generate detailed titration curves for strong acid and strong base reactions. (e) use the titration curve. Titration Curve Ap Chemistry.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Ap Chemistry (e) use the titration curve and the information above to (i). The following might indicate the question deals with buffers and/or titrations: The region where the titrant is added. What i absolutely have to know to survive the ap exam. Effortlessly generate detailed titration curves for strong acid and strong base reactions. The start where the solution only contains either. Titration Curve Ap Chemistry.
From philschatz.com
AcidBase Titrations · Chemistry Titration Curve Ap Chemistry The start where the solution only contains either acid or base. Choosing the best indicator for different titrations depending on the ph at the equivalence. What i absolutely have to know to survive the ap exam. Effortlessly generate detailed titration curves for strong acid and strong base reactions. There are four main points in a titration curve: Ph of the. Titration Curve Ap Chemistry.
From mavink.com
Titration Diagram Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: What i absolutely have to know to survive the ap exam. Effortlessly generate detailed titration curves for strong acid and strong base reactions. The following titration curve shows the change in. The start where the solution only contains either acid or base. Ph of the solution during the titration.. Titration Curve Ap Chemistry.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. The region where the titrant is added. The following titration curve shows the change in. Ph of the solution during the titration. Choosing. Titration Curve Ap Chemistry.
From www.afterskool.com.sg
HL/ H2 Chemistry 101 Titration Curves Summary Guide — AfterSkool Titration Curve Ap Chemistry The start where the solution only contains either acid or base. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. The following might indicate the question deals with buffers and/or titrations: Choosing the best indicator for different titrations depending on the ph at. Titration Curve Ap Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Ap Chemistry The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. There are four main points in a titration curve: (e) use the titration curve and the information above to (i). The following might indicate the question deals with buffers and/or titrations: Ph of the. Titration Curve Ap Chemistry.
From www.showme.com
ap chemistry weak acid/strong base titration curves Science Titration Curve Ap Chemistry Choosing the best indicator for different titrations depending on the ph at the equivalence. The following titration curve shows the change in. The following might indicate the question deals with buffers and/or titrations: The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Effortlessly. Titration Curve Ap Chemistry.
From www.reddit.com
[AP Chem Titration Curves] How do I identify the analyte and titrant Titration Curve Ap Chemistry Effortlessly generate detailed titration curves for strong acid and strong base reactions. (e) use the titration curve and the information above to (i). The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Ph of the solution during the titration. Choosing the best indicator. Titration Curve Ap Chemistry.
From www.youtube.com
AP Chemistry Acid Base Equilibrium Strong Acid and Base Titration Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: There are four main points in a titration curve: The region where the titrant is added. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. What i absolutely have to know to. Titration Curve Ap Chemistry.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Ap Chemistry (e) use the titration curve and the information above to (i). Choosing the best indicator for different titrations depending on the ph at the equivalence. There are four main points in a titration curve: The region where the titrant is added. Effortlessly generate detailed titration curves for strong acid and strong base reactions. Ph of the solution during the titration.. Titration Curve Ap Chemistry.
From www.reddit.com
Upvote that thicc titration curve for ap chem tomorrow r/APStudents Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: (e) use the titration curve and the information above to (i). The start where the solution only contains either acid or base. What i absolutely have to know to survive the ap exam. Ph of the solution during the titration. Choosing the best indicator for different titrations depending on. Titration Curve Ap Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Ap Chemistry The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. What i absolutely have to know to survive the ap exam. Ph of the solution during the titration. Effortlessly generate detailed titration curves for strong acid and strong base reactions. Choosing the best indicator. Titration Curve Ap Chemistry.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Ap Chemistry The following might indicate the question deals with buffers and/or titrations: The region where the titrant is added. Ph of the solution during the titration. Choosing the best indicator for different titrations depending on the ph at the equivalence. There are four main points in a titration curve: What i absolutely have to know to survive the ap exam. (e). Titration Curve Ap Chemistry.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Ap Chemistry The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. The start where the solution only contains either acid or base. The region where the titrant is added. Effortlessly generate detailed titration curves for strong acid and strong base reactions. Ph of the solution. Titration Curve Ap Chemistry.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube Titration Curve Ap Chemistry Choosing the best indicator for different titrations depending on the ph at the equivalence. The region where the titrant is added. The start where the solution only contains either acid or base. The amount of added strong acid or added strong base that a buffer can handle before the ph begins to change appreciably is called the buffer. Ph of. Titration Curve Ap Chemistry.
From www.youtube.com
AP Chemistry Ka Determination from Titration curves YouTube Titration Curve Ap Chemistry The start where the solution only contains either acid or base. The region where the titrant is added. The following might indicate the question deals with buffers and/or titrations: Ph of the solution during the titration. (e) use the titration curve and the information above to (i). There are four main points in a titration curve: The amount of added. Titration Curve Ap Chemistry.
From www.studocu.com
AP Chemistry Acids and Bases NotesTitration Curves AP Chemistry Titration Curve Ap Chemistry Ph of the solution during the titration. There are four main points in a titration curve: The following titration curve shows the change in. The following might indicate the question deals with buffers and/or titrations: (e) use the titration curve and the information above to (i). Choosing the best indicator for different titrations depending on the ph at the equivalence.. Titration Curve Ap Chemistry.