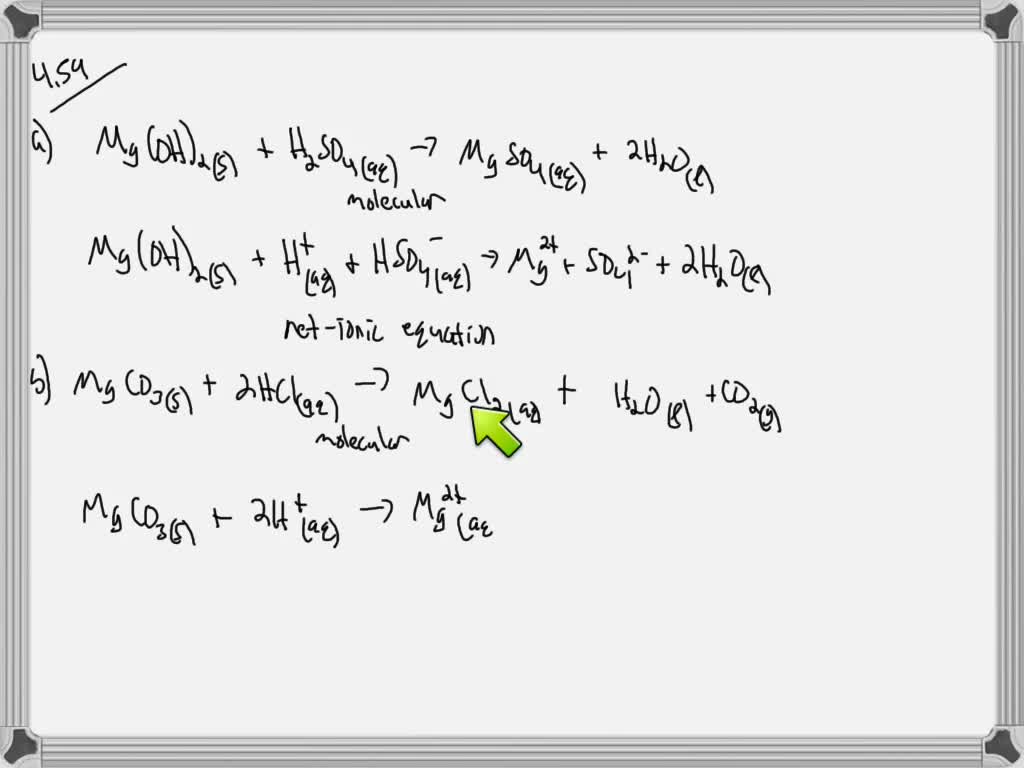Magnesium + Zinc Sulphate Balanced Equation . there are three main steps for writing the net ionic equation for mg +. explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. Zn + mgso4 = mg + zn(so4) is a single displacement. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. let's balance this equation using the inspection method. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. First, we set all coefficients to 1: 1 mg + 1 znso 4 = 1. Zinc + magnesium sulfate = magnesium + zinc sulfate. derive chemical equations from narrative descriptions of chemical reactions.
from gioxcpnsw.blob.core.windows.net
Zn + mgso4 = mg + zn(so4) is a single displacement. explain the roles of subscripts and coefficients in chemical equations. let's balance this equation using the inspection method. Balance a chemical equation when given the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. First, we set all coefficients to 1: there are three main steps for writing the net ionic equation for mg +. 1 mg + 1 znso 4 = 1. Zinc + magnesium sulfate = magnesium + zinc sulfate. Balance a chemical equation when given the.
Magnesium And Zinc Sulfate Net Ionic Equation at Steve Williamson blog
Magnesium + Zinc Sulphate Balanced Equation there are three main steps for writing the net ionic equation for mg +. explain the roles of subscripts and coefficients in chemical equations. Zn + mgso4 = mg + zn(so4) is a single displacement. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. there are three main steps for writing the net ionic equation for mg +. Zinc + magnesium sulfate = magnesium + zinc sulfate. explain the roles of subscripts and coefficients in chemical equations. let's balance this equation using the inspection method. Balance a chemical equation when given the. 1 mg + 1 znso 4 = 1. derive chemical equations from narrative descriptions of chemical reactions. First, we set all coefficients to 1: Balance a chemical equation when given the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the.
From www.numerade.com
SOLVED REPORT SHEET LAB Chemical Reactions and Equations 10 Magnesium + Zinc Sulphate Balanced Equation Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. let's balance this equation using the inspection method. 1 mg + 1 znso 4 = 1. Zinc + magnesium sulfate = magnesium + zinc sulfate. First, we set all coefficients to 1: explain the roles of subscripts and coefficients in chemical equations. . Magnesium + Zinc Sulphate Balanced Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium + Zinc Sulphate Balanced Equation explain the roles of subscripts and coefficients in chemical equations. 1 mg + 1 znso 4 = 1. Balance a chemical equation when given the. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element. Magnesium + Zinc Sulphate Balanced Equation.
From www.numerade.com
SOLVED For each of the word equations convert the given word equations Magnesium + Zinc Sulphate Balanced Equation Balance a chemical equation when given the. derive chemical equations from narrative descriptions of chemical reactions. 1 mg + 1 znso 4 = 1. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. let's balance this equation using the inspection method. Balance a chemical equation. Magnesium + Zinc Sulphate Balanced Equation.
From hxenntpwz.blob.core.windows.net
Magnesium And Zinc Sulphate Equation at Mark Leary blog Magnesium + Zinc Sulphate Balanced Equation 1 mg + 1 znso 4 = 1. Zinc + magnesium sulfate = magnesium + zinc sulfate. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. there are three main steps for writing the net ionic equation for mg +. the chemical equation described in section 4.1 is balanced, meaning that equal. Magnesium + Zinc Sulphate Balanced Equation.
From www.numerade.com
SOLVED Write net ionic equation for the reaction that occurs when Magnesium + Zinc Sulphate Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. Zinc + magnesium sulfate = magnesium + zinc sulfate. derive chemical equations from narrative descriptions of chemical reactions. . Magnesium + Zinc Sulphate Balanced Equation.
From exyfbegni.blob.core.windows.net
Magnesium And Zinc Chloride Balanced Equation at Jermaine Nixon blog Magnesium + Zinc Sulphate Balanced Equation there are three main steps for writing the net ionic equation for mg +. Balance a chemical equation when given the. let's balance this equation using the inspection method. Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. Consider the balanced chemical equation shown below for the reaction of. Magnesium + Zinc Sulphate Balanced Equation.
From www.youtube.com
MgSO4+NaOH=Mg(OH)2+Na2SO4. balance the chemical equation Magnesium + Zinc Sulphate Balanced Equation explain the roles of subscripts and coefficients in chemical equations. explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. derive chemical equations from narrative descriptions of chemical reactions. Zn + mgso4 = mg +. Magnesium + Zinc Sulphate Balanced Equation.
From www.youtube.com
H2SO4+Mg(OH)2=H2O+MgSO4 Balanced EquationSulphuric Acid+Magnesium Magnesium + Zinc Sulphate Balanced Equation 1 mg + 1 znso 4 = 1. Balance a chemical equation when given the. derive chemical equations from narrative descriptions of chemical reactions. First, we set all coefficients to 1: Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. let's balance this equation using the inspection method. Zinc + magnesium sulfate. Magnesium + Zinc Sulphate Balanced Equation.
From www.toppr.com
Write a balanced chemical equation with the help of chemical symbols Magnesium + Zinc Sulphate Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Zn + mgso4 = mg + zn(so4) is a single displacement. Zinc + magnesium sulfate = magnesium + zinc sulfate. First, we set all coefficients to 1: derive chemical equations from narrative descriptions of chemical reactions. . Magnesium + Zinc Sulphate Balanced Equation.
From www.chegg.com
Solved Translate Each Word Equation into a Balanced Chemical Magnesium + Zinc Sulphate Balanced Equation Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. explain the roles of subscripts and coefficients in chemical equations. derive chemical equations from narrative descriptions of chemical reactions. 1 mg + 1 znso 4 = 1. let's balance this equation using the inspection method. Zinc + magnesium sulfate = magnesium +. Magnesium + Zinc Sulphate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equations 1 zinc react with Magnesium + Zinc Sulphate Balanced Equation Balance a chemical equation when given the. derive chemical equations from narrative descriptions of chemical reactions. explain the roles of subscripts and coefficients in chemical equations. Zinc + magnesium sulfate = magnesium + zinc sulfate. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. Balance a chemical equation when given the. Zn. Magnesium + Zinc Sulphate Balanced Equation.
From www.chegg.com
Solved 14.Which of the following is the properly balanced Magnesium + Zinc Sulphate Balanced Equation there are three main steps for writing the net ionic equation for mg +. let's balance this equation using the inspection method. Balance a chemical equation when given the. First, we set all coefficients to 1: Zn + mgso4 = mg + zn(so4) is a single displacement. derive chemical equations from narrative descriptions of chemical reactions. Balance. Magnesium + Zinc Sulphate Balanced Equation.
From www.numerade.com
SOLVED magnesium sulfate and potassium phosphate (potassium produces Magnesium + Zinc Sulphate Balanced Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. Balance a chemical equation when given the. 1 mg + 1 znso 4 = 1. there are three main steps for writing the net ionic equation for mg +. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in. Magnesium + Zinc Sulphate Balanced Equation.
From chemisthunter.com
Zn + MgSO4 = Mg + ZnSO4 Magnesium + Zinc Sulphate Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Zn + mgso4 = mg + zn(so4) is a single displacement. explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. let's balance this equation using the inspection method.. Magnesium + Zinc Sulphate Balanced Equation.
From www.toppr.com
Write a balanced chemical equation with the help of chemical symbols Magnesium + Zinc Sulphate Balanced Equation 1 mg + 1 znso 4 = 1. Zinc + magnesium sulfate = magnesium + zinc sulfate. Balance a chemical equation when given the. Zn + mgso4 = mg + zn(so4) is a single displacement. let's balance this equation using the inspection method. explain the roles of subscripts and coefficients in chemical equations. there are three main. Magnesium + Zinc Sulphate Balanced Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium + Zinc Sulphate Balanced Equation derive chemical equations from narrative descriptions of chemical reactions. Zn + mgso4 = mg + zn(so4) is a single displacement. First, we set all coefficients to 1: Balance a chemical equation when given the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. explain the. Magnesium + Zinc Sulphate Balanced Equation.
From gioxcpnsw.blob.core.windows.net
Magnesium And Zinc Sulfate Net Ionic Equation at Steve Williamson blog Magnesium + Zinc Sulphate Balanced Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. explain the roles of subscripts and coefficients in chemical equations. Zn + mgso4 = mg + zn(so4) is a single displacement. explain the roles of subscripts and. Magnesium + Zinc Sulphate Balanced Equation.
From www.numerade.com
SOLVED Question 3 40 pts Write balanced chemical equations from the Magnesium + Zinc Sulphate Balanced Equation there are three main steps for writing the net ionic equation for mg +. Balance a chemical equation when given the. Balance a chemical equation when given the. Zinc + magnesium sulfate = magnesium + zinc sulfate. let's balance this equation using the inspection method. Zn + mgso4 = mg + zn(so4) is a single displacement. Consider the. Magnesium + Zinc Sulphate Balanced Equation.
From www.numerade.com
SOLVED Question 2 [25 pts] a) [15 pts] Write balanced Magnesium + Zinc Sulphate Balanced Equation 1 mg + 1 znso 4 = 1. explain the roles of subscripts and coefficients in chemical equations. First, we set all coefficients to 1: Zinc + magnesium sulfate = magnesium + zinc sulfate. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Consider the balanced. Magnesium + Zinc Sulphate Balanced Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium + Zinc Sulphate Balanced Equation Balance a chemical equation when given the. Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. there are three main steps for writing the net ionic equation for mg +. Zn + mgso4 = mg + zn(so4) is a single displacement. Zinc + magnesium sulfate = magnesium + zinc sulfate.. Magnesium + Zinc Sulphate Balanced Equation.
From www.toppr.com
Convert the following word equations into molecular equation and Magnesium + Zinc Sulphate Balanced Equation let's balance this equation using the inspection method. Zinc + magnesium sulfate = magnesium + zinc sulfate. 1 mg + 1 znso 4 = 1. explain the roles of subscripts and coefficients in chemical equations. derive chemical equations from narrative descriptions of chemical reactions. Balance a chemical equation when given the. Balance a chemical equation when given. Magnesium + Zinc Sulphate Balanced Equation.
From www.youtube.com
How to Write the Formula for Magnesium sulfate YouTube Magnesium + Zinc Sulphate Balanced Equation explain the roles of subscripts and coefficients in chemical equations. derive chemical equations from narrative descriptions of chemical reactions. Zinc + magnesium sulfate = magnesium + zinc sulfate. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. let's balance this equation using the inspection. Magnesium + Zinc Sulphate Balanced Equation.
From hxekwfnye.blob.core.windows.net
Magnesium + Zinc Sulphate Equation at Jimmy Walker blog Magnesium + Zinc Sulphate Balanced Equation First, we set all coefficients to 1: derive chemical equations from narrative descriptions of chemical reactions. 1 mg + 1 znso 4 = 1. Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. Zinc + magnesium sulfate = magnesium + zinc sulfate. Consider the balanced chemical equation shown below for. Magnesium + Zinc Sulphate Balanced Equation.
From hxenntpwz.blob.core.windows.net
Magnesium And Zinc Sulphate Equation at Mark Leary blog Magnesium + Zinc Sulphate Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. explain the roles of subscripts and coefficients in chemical equations. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. Zinc + magnesium sulfate = magnesium + zinc sulfate. Balance a chemical. Magnesium + Zinc Sulphate Balanced Equation.
From brainly.in
Give balanced equation A to D for the following conversions Zinc → Magnesium + Zinc Sulphate Balanced Equation First, we set all coefficients to 1: let's balance this equation using the inspection method. derive chemical equations from narrative descriptions of chemical reactions. Balance a chemical equation when given the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Balance a chemical equation when. Magnesium + Zinc Sulphate Balanced Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Magnesium + Zinc Sulphate Balanced Equation Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. explain the roles of subscripts and coefficients in chemical equations. let's balance this equation using the inspection method. Zinc + magnesium sulfate = magnesium + zinc sulfate. Zn + mgso4 = mg + zn(so4) is a single displacement. there are three main. Magnesium + Zinc Sulphate Balanced Equation.
From www.youtube.com
Equation for MgSO4 + H2O (Magnesium sulfate + Water) YouTube Magnesium + Zinc Sulphate Balanced Equation Balance a chemical equation when given the. there are three main steps for writing the net ionic equation for mg +. Zn + mgso4 = mg + zn(so4) is a single displacement. let's balance this equation using the inspection method. explain the roles of subscripts and coefficients in chemical equations. the chemical equation described in section. Magnesium + Zinc Sulphate Balanced Equation.
From dayanara-blogmosley.blogspot.com
Magnesium Sulfate and Sodium Carbonate Balanced Equation Magnesium + Zinc Sulphate Balanced Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. let's balance this equation using the inspection method. Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. 1 mg + 1 znso 4 = 1. First, we set all coefficients to 1: Consider the balanced chemical equation shown below for the. Magnesium + Zinc Sulphate Balanced Equation.
From giogbvdps.blob.core.windows.net
Magnesium And Zinc Reactivity at Bruce Neal blog Magnesium + Zinc Sulphate Balanced Equation 1 mg + 1 znso 4 = 1. Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. explain the roles of subscripts and coefficients in chemical equations. explain the roles of subscripts and coefficients in chemical equations. derive chemical equations from narrative descriptions of chemical reactions. Zn + mgso4 = mg. Magnesium + Zinc Sulphate Balanced Equation.
From www.toppr.com
Convert the following word equations into molecular equation and Magnesium + Zinc Sulphate Balanced Equation First, we set all coefficients to 1: Zinc + magnesium sulfate = magnesium + zinc sulfate. Zn + mgso4 = mg + zn(so4) is a single displacement. derive chemical equations from narrative descriptions of chemical reactions. let's balance this equation using the inspection method. the chemical equation described in section 4.1 is balanced, meaning that equal numbers. Magnesium + Zinc Sulphate Balanced Equation.
From brainly.in
What is the ionic equation for magnesium + zinc sulphate gives Magnesium + Zinc Sulphate Balanced Equation Balance a chemical equation when given the. Zinc + magnesium sulfate = magnesium + zinc sulfate. Balance a chemical equation when given the. let's balance this equation using the inspection method. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Consider the balanced chemical equation shown. Magnesium + Zinc Sulphate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + ZnSO4 = Zn + MgSO4 (See Magnesium + Zinc Sulphate Balanced Equation explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the. Balance a chemical equation when given the. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. there are three main steps for writing the net ionic equation for. Magnesium + Zinc Sulphate Balanced Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Magnesium + Zinc Sulphate Balanced Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. derive chemical equations from narrative descriptions of chemical reactions. Balance a chemical equation when given the. explain the roles of subscripts and coefficients in chemical equations. there are three main steps for writing the net ionic equation for mg +. First, we set all coefficients to 1: Balance. Magnesium + Zinc Sulphate Balanced Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation showing the reaction between Magnesium + Zinc Sulphate Balanced Equation Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. derive chemical equations from narrative descriptions of chemical reactions. First, we set all coefficients to 1: the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Zn + mgso4 = mg +. Magnesium + Zinc Sulphate Balanced Equation.
From www.toppr.com
Write a balanced chemical equation with the help of chemical symbols Magnesium + Zinc Sulphate Balanced Equation Consider the balanced chemical equation shown below for the reaction of solid magnesium with aqueous. Zn + mgso4 = mg + zn(so4) is a single displacement. Zinc + magnesium sulfate = magnesium + zinc sulfate. explain the roles of subscripts and coefficients in chemical equations. explain the roles of subscripts and coefficients in chemical equations. let's balance. Magnesium + Zinc Sulphate Balanced Equation.