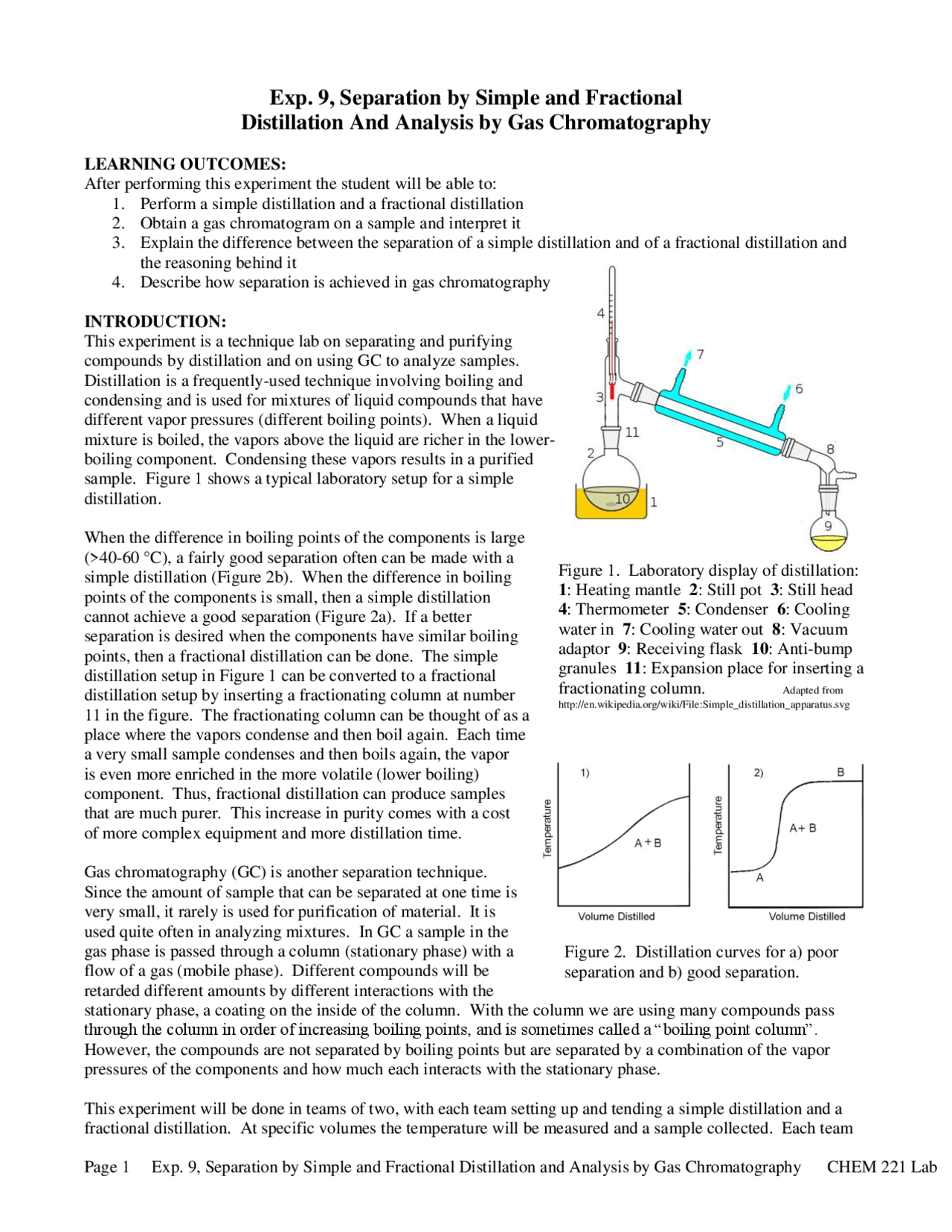
Distillation Lab Procedure . One where steam is directly generated by heating water in the distilling flask with a. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. Collect distillate at a rate of 1 drop. Turn on the condenser water. Apply the heat source to the distilling flask. distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. two variations of steam distillation are regularly used: Simple distillation is a procedure by which two liquids with different boiling points can be separated. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere.
from www.docsity.com
two variations of steam distillation are regularly used: distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Apply the heat source to the distilling flask. Simple distillation is a procedure by which two liquids with different boiling points can be separated. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. Collect distillate at a rate of 1 drop. One where steam is directly generated by heating water in the distilling flask with a. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Turn on the condenser water.
Simple and fractional distillation lab report Study Guides, Projects
Distillation Lab Procedure Simple distillation is a procedure by which two liquids with different boiling points can be separated. One where steam is directly generated by heating water in the distilling flask with a. Simple distillation is a procedure by which two liquids with different boiling points can be separated. two variations of steam distillation are regularly used: the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. Collect distillate at a rate of 1 drop. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Apply the heat source to the distilling flask. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. Turn on the condenser water. distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids.
From chem.libretexts.org
5.2C StepbyStep Procedures Chemistry LibreTexts Distillation Lab Procedure One where steam is directly generated by heating water in the distilling flask with a. Simple distillation is a procedure by which two liquids with different boiling points can be separated. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. Turn on the condenser water.. Distillation Lab Procedure.
From www.shutterstock.com
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous Distillation Lab Procedure Simple distillation is a procedure by which two liquids with different boiling points can be separated. One where steam is directly generated by heating water in the distilling flask with a. Turn on the condenser water. Apply the heat source to the distilling flask. distillation is one of the oldest and still most common methods for both the purification. Distillation Lab Procedure.
From exyktwziu.blob.core.windows.net
Distillation Experiment Procedure at Victor Amaro blog Distillation Lab Procedure in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Simple distillation is a procedure by which two liquids with different boiling points can be separated. two variations of steam distillation are regularly used: distillation is one of the oldest and still most common methods for both the. Distillation Lab Procedure.
From mavink.com
Labelled Diagram Of Distillation Distillation Lab Procedure One where steam is directly generated by heating water in the distilling flask with a. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. Apply the heat source to the distilling flask. distillation is the process of heating a liquid until it boils, then. Distillation Lab Procedure.
From www.pinterest.com
Simple Distillation Distillation, Chemistry lessons, Teaching chemistry Distillation Lab Procedure distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. distillation is the process of heating a liquid until it boils, then condensing. Distillation Lab Procedure.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Distillation Lab Procedure Apply the heat source to the distilling flask. One where steam is directly generated by heating water in the distilling flask with a. Collect distillate at a rate of 1 drop. two variations of steam distillation are regularly used: Simple distillation is a procedure by which two liquids with different boiling points can be separated. distillation is one. Distillation Lab Procedure.
From studylib.net
Experiment 2. Separation of Liquids by Distillation. Distillation Lab Procedure Apply the heat source to the distilling flask. Turn on the condenser water. two variations of steam distillation are regularly used: Collect distillate at a rate of 1 drop. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. One where steam is directly generated by heating water in. Distillation Lab Procedure.
From studylib.net
Steam Distillation Experiment Distillation Lab Procedure distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. two variations of steam distillation are regularly used: One where steam is directly generated by heating water in the distilling flask with a. in the simplest terms, a distillation involves boiling a liquid, then condensing the. Distillation Lab Procedure.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Distillation Lab Procedure Simple distillation is a procedure by which two liquids with different boiling points can be separated. Turn on the condenser water. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. Collect distillate at a rate of 1 drop. two variations of steam distillation are. Distillation Lab Procedure.
From www.vedantu.com
Distillation What Is Distillation? Process and Uses of Distillation Distillation Lab Procedure Collect distillate at a rate of 1 drop. distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Turn on the condenser water. two variations of steam distillation are regularly used: One where steam is directly generated by heating water in the distilling flask with a. . Distillation Lab Procedure.
From en.wikipedia.org
Distillation Wikipedia Distillation Lab Procedure Apply the heat source to the distilling flask. distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Simple distillation is a procedure by which two liquids with different boiling points can be separated. distillation is the process of heating a liquid until it boils, then condensing. Distillation Lab Procedure.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Distillation Lab Procedure Collect distillate at a rate of 1 drop. Turn on the condenser water. One where steam is directly generated by heating water in the distilling flask with a. Simple distillation is a procedure by which two liquids with different boiling points can be separated. two variations of steam distillation are regularly used: the use of distillation to separate. Distillation Lab Procedure.
From www.thoughtco.com
What Is Distillation? Principles and Uses Distillation Lab Procedure the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. One where steam is directly generated by heating water in the distilling flask with a. two variations of steam distillation are regularly used: distillation is the process of heating a liquid until it boils,. Distillation Lab Procedure.
From aimhigh.space
Distillation and Chromatography AimHigh! Distillation Lab Procedure Apply the heat source to the distilling flask. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. One where steam is directly generated by heating water in the distilling flask with a. the use of distillation to separate the components in a mixture is based on the principle. Distillation Lab Procedure.
From mavink.com
Fractional Distillation Labeled Diagram Distillation Lab Procedure Apply the heat source to the distilling flask. Simple distillation is a procedure by which two liquids with different boiling points can be separated. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is the process of heating a liquid until it boils, then condensing and collecting. Distillation Lab Procedure.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Lab Procedure two variations of steam distillation are regularly used: Simple distillation is a procedure by which two liquids with different boiling points can be separated. Turn on the condenser water. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. distillation is one of the oldest and still most. Distillation Lab Procedure.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Distillation Lab Procedure two variations of steam distillation are regularly used: One where steam is directly generated by heating water in the distilling flask with a. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours.. Distillation Lab Procedure.
From www.youtube.com
Introduction to Fractional distillation Distillation procedure Home Distillation Lab Procedure in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Turn on the condenser water. Simple distillation is a procedure by which two liquids with different boiling points can be separated. two variations of steam distillation are regularly used: distillation is the process of heating a liquid until. Distillation Lab Procedure.
From studylib.net
Distillation Lab Distillation Lab Procedure distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. the use of distillation to separate the components in a mixture is based on the principle that. Distillation Lab Procedure.
From www.vrogue.co
Sequence Of Distillation Process Download Scientific vrogue.co Distillation Lab Procedure One where steam is directly generated by heating water in the distilling flask with a. Simple distillation is a procedure by which two liquids with different boiling points can be separated. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Collect distillate at a rate of 1 drop. Apply. Distillation Lab Procedure.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Distillation Lab Procedure distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. two variations of steam distillation are regularly used: the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. Turn on the condenser water.. Distillation Lab Procedure.
From help.chemix.org
Drawing distillation setups Chemix Help Center Distillation Lab Procedure in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. One where steam is directly generated by heating water in the distilling flask with a. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. two variations of steam. Distillation Lab Procedure.
From studylib.net
Simple Distillation Distillation Lab Procedure distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. Simple distillation is a procedure by which two liquids with different boiling points can be separated. Collect distillate at a rate of 1 drop. Apply the heat source to the distilling flask. One where steam is directly generated by heating. Distillation Lab Procedure.
From exyktwziu.blob.core.windows.net
Distillation Experiment Procedure at Victor Amaro blog Distillation Lab Procedure distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Turn on the condenser water. One where steam is directly generated by heating water in the distilling flask. Distillation Lab Procedure.
From www.nagwa.com
Question Video Identifying a Piece of Apparatus Used in Fractional Distillation Lab Procedure the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. Collect distillate at a rate of 1 drop. distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Turn on the condenser water. . Distillation Lab Procedure.
From www.goldhilleducation.co.uk
simple distillation Distillation Lab Procedure the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. two variations of steam distillation are regularly used: in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is one of the oldest. Distillation Lab Procedure.
From pharmacygyan.com
Fractional distillation Principle Construction Working etc.. Pharmacy Gyan Distillation Lab Procedure Collect distillate at a rate of 1 drop. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. One where steam is directly generated by heating. Distillation Lab Procedure.
From www.docsity.com
Simple and fractional distillation lab report Study Guides, Projects Distillation Lab Procedure Turn on the condenser water. Collect distillate at a rate of 1 drop. Apply the heat source to the distilling flask. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. One where steam is directly generated by heating water in the distilling flask with a. distillation is the. Distillation Lab Procedure.
From www.etsy.com
Distillation Apparatus Diagram Poster Printable Lab Tools Etsy Distillation Lab Procedure Collect distillate at a rate of 1 drop. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. distillation is one of the oldest and still most common methods. Distillation Lab Procedure.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Distillation Lab Procedure in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Turn on the condenser water. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. the use of distillation to separate the components in a mixture is based on. Distillation Lab Procedure.
From chem.libretexts.org
5.3D StepbyStep Procedures for Fractional Distillation Chemistry Distillation Lab Procedure One where steam is directly generated by heating water in the distilling flask with a. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. Simple distillation is a procedure by which two liquids with different boiling points can be separated. distillation is one of the oldest and still. Distillation Lab Procedure.
From exyktwziu.blob.core.windows.net
Distillation Experiment Procedure at Victor Amaro blog Distillation Lab Procedure distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. Turn on the condenser water. Collect distillate at a rate of 1 drop. the use of distillation to separate. Distillation Lab Procedure.
From www.jove.com
Fractional Distillation Principle, Purification of a Mixture Organic Distillation Lab Procedure in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. the use of distillation to separate the components in a mixture is based on the principle that the liquid in the boiling flask. distillation is the process of heating a liquid until it boils, then condensing and collecting. Distillation Lab Procedure.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Distillation Lab Procedure Apply the heat source to the distilling flask. distillation is the process of heating a liquid until it boils, then condensing and collecting the resultant hot vapours. Turn on the condenser water. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is one of the oldest. Distillation Lab Procedure.
From www.shutterstock.com
Distillation Method RoyaltyFree Images, Stock Photos & Pictures Distillation Lab Procedure One where steam is directly generated by heating water in the distilling flask with a. two variations of steam distillation are regularly used: Apply the heat source to the distilling flask. in the simplest terms, a distillation involves boiling a liquid, then condensing the gas and collecting the liquid elsewhere. distillation is the process of heating a. Distillation Lab Procedure.