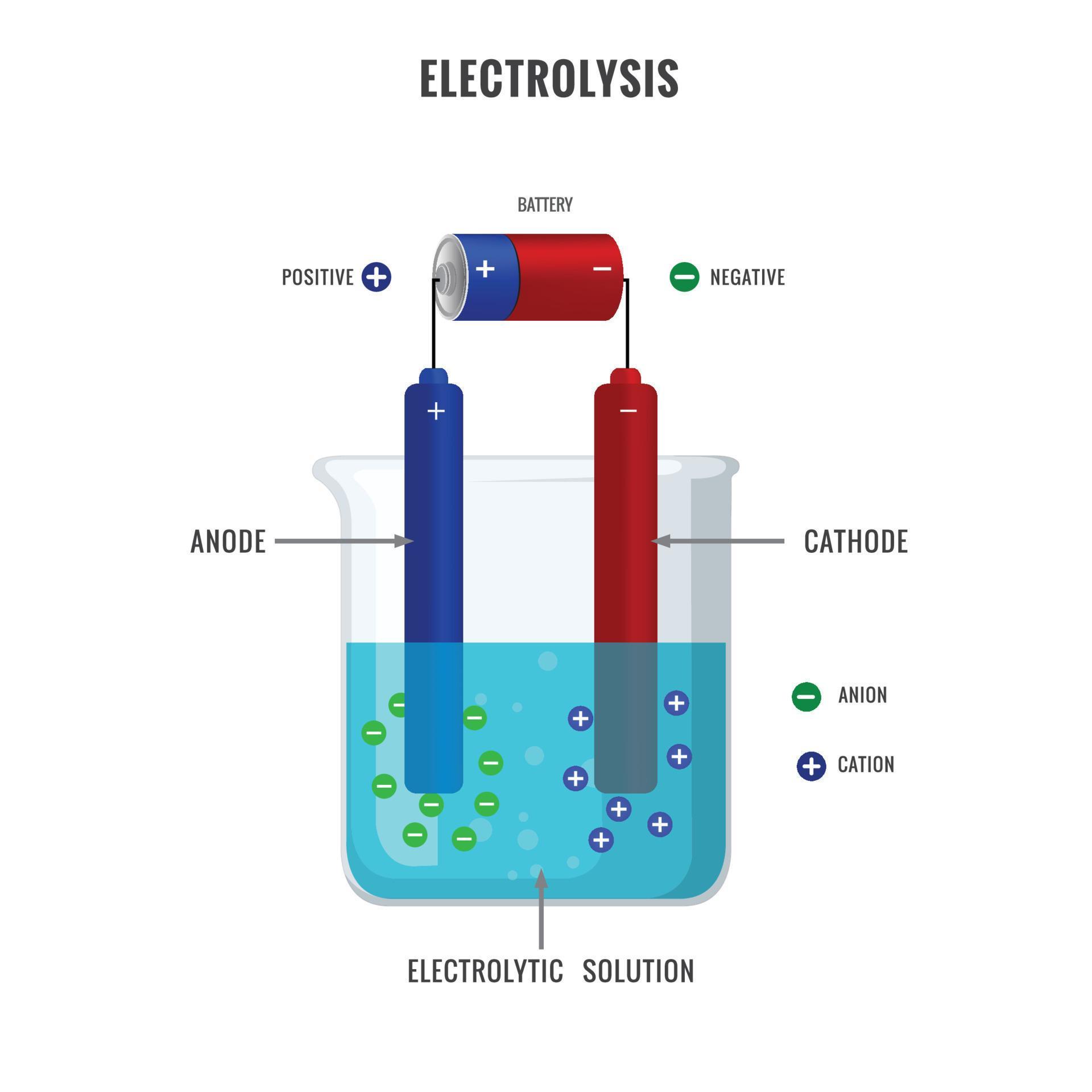
Electrolyte Solution For Electrolysis . For transition metal oxides, the oer. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. This page looks at the electrolysis of aqueous solutions of compounds. Most people will have met quite a lot. i am looking for an electrolyte to use in water to perform electrolysis. the electrolysis of solutions. Ionic compounds conduct electricity when molten or in. It uses a direct current (dc). reactive metals are extracted from their ores using electrolysis. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. It can't be salt, as salt ($\ce {nacl}$) would.
from www.vecteezy.com
Most people will have met quite a lot. For transition metal oxides, the oer. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. It can't be salt, as salt ($\ce {nacl}$) would. It uses a direct current (dc). the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. Ionic compounds conduct electricity when molten or in. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. the electrolysis of solutions. i am looking for an electrolyte to use in water to perform electrolysis.
Electrolysis of electrolyte solution in electrochemistry vector
Electrolyte Solution For Electrolysis i am looking for an electrolyte to use in water to perform electrolysis. It uses a direct current (dc). It can't be salt, as salt ($\ce {nacl}$) would. This page looks at the electrolysis of aqueous solutions of compounds. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. i am looking for an electrolyte to use in water to perform electrolysis. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. Most people will have met quite a lot. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. the electrolysis of solutions. For transition metal oxides, the oer.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Electrolyte Solution For Electrolysis the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. Most people will have met quite a lot. It uses a direct current (dc). electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. the electrolysis. Electrolyte Solution For Electrolysis.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Electrolyte Solution For Electrolysis Most people will have met quite a lot. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. the electrolysis of solutions. For transition metal oxides, the oer. Ionic compounds conduct electricity when molten. Electrolyte Solution For Electrolysis.
From www.adevos.science
Electrolysis Adevoscience Electrolyte Solution For Electrolysis the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. It can't be salt, as salt ($\ce {nacl}$) would. For transition metal oxides, the oer. It uses a direct current (dc). Ionic compounds conduct electricity when molten or in. i am looking for an electrolyte to use in water to perform. Electrolyte Solution For Electrolysis.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Electrolyte Solution For Electrolysis the electrolysis of solutions. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. Ionic compounds conduct electricity when molten or in. This page looks at the electrolysis of aqueous solutions of compounds. reactive metals are extracted from their ores using electrolysis. . Electrolyte Solution For Electrolysis.
From pandai.me
Electrolytic Cell Electrolyte Solution For Electrolysis reactive metals are extracted from their ores using electrolysis. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. It can't be salt, as salt ($\ce {nacl}$) would. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. Most people will have met quite a. Electrolyte Solution For Electrolysis.
From www.slideserve.com
PPT SOLUTIONS OF ELECTROLYTES PowerPoint Presentation, free download Electrolyte Solution For Electrolysis the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. This page looks at the electrolysis of aqueous solutions of compounds. Ionic compounds conduct electricity when molten or in. Most people will have met quite a lot. reactive metals are extracted from their ores using electrolysis. i am looking for. Electrolyte Solution For Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrolyte Solution For Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. Most people will have met quite a lot. i am looking for an electrolyte to use in water to perform electrolysis. electrolysis separates chemically bonded ionic substances and compounds by passing an electric. Electrolyte Solution For Electrolysis.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrolyte Solution For Electrolysis Ionic compounds conduct electricity when molten or in. It can't be salt, as salt ($\ce {nacl}$) would. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. For transition metal oxides, the oer. reactive metals are extracted from their ores using electrolysis. Most people. Electrolyte Solution For Electrolysis.
From philschatz.com
Electrolysis · Chemistry Electrolyte Solution For Electrolysis For transition metal oxides, the oer. It can't be salt, as salt ($\ce {nacl}$) would. i am looking for an electrolyte to use in water to perform electrolysis. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in. Electrolyte Solution For Electrolysis.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrolyte Solution For Electrolysis Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. This page looks at the electrolysis of aqueous solutions of compounds. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. Most people will have met quite a lot. electrolysis separates chemically bonded. Electrolyte Solution For Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrolyte Solution For Electrolysis the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. reactive metals are extracted from their ores using electrolysis. It uses a direct current (dc). It can't be salt, as salt ($\ce {nacl}$) would. Ionic. Electrolyte Solution For Electrolysis.
From studylib.net
Electrolysis of solutions Electrolyte Solution For Electrolysis This page looks at the electrolysis of aqueous solutions of compounds. It can't be salt, as salt ($\ce {nacl}$) would. Ionic compounds conduct electricity when molten or in. i am looking for an electrolyte to use in water to perform electrolysis. reactive metals are extracted from their ores using electrolysis. the electrolyte provides a pathway for ions. Electrolyte Solution For Electrolysis.
From www.elevise.co.uk
C4 L) Electrolysis Part 2 AQA Chemistry Elevise Electrolyte Solution For Electrolysis It uses a direct current (dc). Ionic compounds conduct electricity when molten or in. Most people will have met quite a lot. This page looks at the electrolysis of aqueous solutions of compounds. reactive metals are extracted from their ores using electrolysis. i am looking for an electrolyte to use in water to perform electrolysis. electrolysis separates. Electrolyte Solution For Electrolysis.
From www.alamy.com
Flat illustration of electrolysis of electrolyte solution in Electrolyte Solution For Electrolysis For transition metal oxides, the oer. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. This page looks at the electrolysis of aqueous solutions of compounds. It can't be salt, as salt ($\ce {nacl}$) would. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy. Electrolyte Solution For Electrolysis.
From www.vecteezy.com
Electrolysis of electrolyte solution, Simple electrolysis process of an Electrolyte Solution For Electrolysis reactive metals are extracted from their ores using electrolysis. For transition metal oxides, the oer. i am looking for an electrolyte to use in water to perform electrolysis. the electrolysis of solutions. This page looks at the electrolysis of aqueous solutions of compounds. Ionic compounds conduct electricity when molten or in. It can't be salt, as salt. Electrolyte Solution For Electrolysis.
From circuitlibscombrid.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrolyte Solution For Electrolysis the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. Most people will have met quite a lot. reactive metals are extracted from their ores using electrolysis. For transition metal oxides, the oer. the electrolysis of solutions. It can't be salt, as salt ($\ce {nacl}$) would. electrolysis can occur. Electrolyte Solution For Electrolysis.
From www.researchgate.net
Pure electrolysis of different electrolyte solutions and expansion of Electrolyte Solution For Electrolysis the electrolysis of solutions. i am looking for an electrolyte to use in water to perform electrolysis. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. It can't be salt, as salt ($\ce {nacl}$) would. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy. Electrolyte Solution For Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrolyte Solution For Electrolysis electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. This page looks at the electrolysis of aqueous solutions of compounds. Most people will have met quite a lot. It can't be salt, as salt ($\ce {nacl}$) would. the electrolysis of solutions. electrolysis can occur in electrolytic cells by introducing a power. Electrolyte Solution For Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrolyte Solution For Electrolysis It can't be salt, as salt ($\ce {nacl}$) would. This page looks at the electrolysis of aqueous solutions of compounds. It uses a direct current (dc). electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. Most people will have met quite a lot. i am looking for an electrolyte to use in. Electrolyte Solution For Electrolysis.
From library.fiveable.me
Electrolysis Intro to Chemistry Class Notes Fiveable Electrolyte Solution For Electrolysis For transition metal oxides, the oer. reactive metals are extracted from their ores using electrolysis. It uses a direct current (dc). It can't be salt, as salt ($\ce {nacl}$) would. Most people will have met quite a lot. This page looks at the electrolysis of aqueous solutions of compounds. i am looking for an electrolyte to use in. Electrolyte Solution For Electrolysis.
From shop.wf-education.com
Understanding Electrolysis Electrolyte Solution For Electrolysis Most people will have met quite a lot. the electrolysis of solutions. It uses a direct current (dc). For transition metal oxides, the oer. It can't be salt, as salt ($\ce {nacl}$) would. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. This page looks at the electrolysis of aqueous solutions of. Electrolyte Solution For Electrolysis.
From fyooalhxu.blob.core.windows.net
How Do You Make Electrolyte Solution at Deborah Pellerin blog Electrolyte Solution For Electrolysis It uses a direct current (dc). the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. Ionic compounds conduct electricity when molten or in. Most people will have met quite a lot. It can't be salt, as salt ($\ce {nacl}$) would. electrolysis separates chemically bonded ionic substances and compounds by passing. Electrolyte Solution For Electrolysis.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry Electrolyte Solution For Electrolysis Most people will have met quite a lot. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. It uses a direct current (dc). It can't be salt, as salt ($\ce {nacl}$) would. i am looking for an electrolyte to use in water to. Electrolyte Solution For Electrolysis.
From foodnurish.com
6 Easy Methods On How To Replenish Electrolytes (+Recipes) Electrolyte Solution For Electrolysis For transition metal oxides, the oer. It uses a direct current (dc). the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. i am looking for. Electrolyte Solution For Electrolysis.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki Electrolyte Solution For Electrolysis For transition metal oxides, the oer. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. This page looks at the electrolysis of aqueous solutions of compounds. i am looking for an electrolyte to. Electrolyte Solution For Electrolysis.
From hydrofluxwelder.com
HF08 Electrolyte Solution Electrolyte Solution For Electrolysis electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. Most people will have met quite a lot. This page looks at the electrolysis of aqueous solutions of compounds. It uses a direct current (dc). Ionic compounds conduct electricity when molten or in. the electrolysis of solutions. For transition metal oxides, the oer.. Electrolyte Solution For Electrolysis.
From in.pinterest.com
What is Electrolysis and Electrolyte with examples. Science fair Electrolyte Solution For Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. This page looks at the electrolysis of aqueous solutions of compounds. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. i am looking for an electrolyte to. Electrolyte Solution For Electrolysis.
From www.alamy.com
Electrolysis of water diagram. Battery, anode, cathode, cation, anion Electrolyte Solution For Electrolysis electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. reactive metals are extracted from their ores using electrolysis. It can't be salt, as salt ($\ce {nacl}$) would. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. For transition metal oxides, the oer. Ionic. Electrolyte Solution For Electrolysis.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Electrolyte Solution For Electrolysis Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. This page looks at the electrolysis of aqueous solutions of compounds. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the. Electrolyte Solution For Electrolysis.
From www.vecteezy.com
Electrolysis of electrolyte solution in electrochemistry vector Electrolyte Solution For Electrolysis It can't be salt, as salt ($\ce {nacl}$) would. the electrolysis of solutions. For transition metal oxides, the oer. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. Ionic compounds conduct electricity when molten. Electrolyte Solution For Electrolysis.
From www.dreamstime.com
Electrolysis. Experimental Set Up for Electrolysis Stock Vector Electrolyte Solution For Electrolysis reactive metals are extracted from their ores using electrolysis. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. Ionic compounds conduct electricity when molten or. Electrolyte Solution For Electrolysis.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolyte Solution For Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. reactive metals are extracted from their ores using electrolysis. electrolysis separates chemically bonded ionic substances. Electrolyte Solution For Electrolysis.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process Electrolyte Solution For Electrolysis the electrolyte provides a pathway for ions to stream between the electrodes and maintains the charge balance. Ionic compounds conduct electricity when molten or in. For transition metal oxides, the oer. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the nonspontaneous. electrolysis separates. Electrolyte Solution For Electrolysis.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrolyte Solution For Electrolysis reactive metals are extracted from their ores using electrolysis. i am looking for an electrolyte to use in water to perform electrolysis. For transition metal oxides, the oer. electrolysis separates chemically bonded ionic substances and compounds by passing an electric current through them. It can't be salt, as salt ($\ce {nacl}$) would. Ionic compounds conduct electricity when. Electrolyte Solution For Electrolysis.
From www.researchgate.net
Comparison of electrolysis of different electrolyte solution (water Electrolyte Solution For Electrolysis i am looking for an electrolyte to use in water to perform electrolysis. For transition metal oxides, the oer. the electrolysis of solutions. It uses a direct current (dc). It can't be salt, as salt ($\ce {nacl}$) would. Most people will have met quite a lot. electrolysis can occur in electrolytic cells by introducing a power supply,. Electrolyte Solution For Electrolysis.