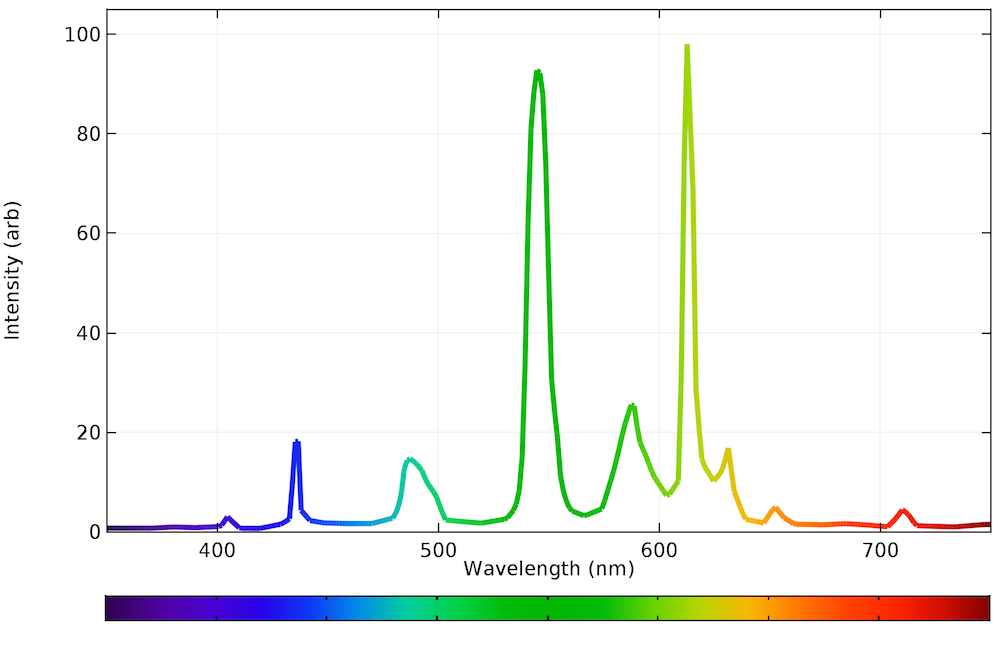
Spectroscopy Of Emission . the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a.
from www.comsol.com
atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. 10.7.1 atomic emission spectra. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a.
Calculating the Emission Spectra from Common Light Sources COMSOL Blog
Spectroscopy Of Emission 10.7.1 atomic emission spectra. 10.7.1 atomic emission spectra. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a.
From www.priyamstudycentre.com
Atomic Emission Spectroscopy Inductively Coupled Plasma Spectroscopy Of Emission the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. atomic emission spectroscopy (aes) is a method of chemical analysis that. Spectroscopy Of Emission.
From www.slideserve.com
PPT Principle of Emission Spectroscopy I PowerPoint Presentation, free download ID2250109 Spectroscopy Of Emission 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. The emission spectrum is the set of light frequencies emitted. Spectroscopy Of Emission.
From www.slideserve.com
PPT Principle of Emission Spectroscopy I PowerPoint Presentation, free download ID2250109 Spectroscopy Of Emission 10.7.1 atomic emission spectra. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. Atomic emission occurs when a valence electron in a. Spectroscopy Of Emission.
From physicsopenlab.org
Arc Atomic Emission Spectroscopy PhysicsOpenLab Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. 10.7.1 atomic emission spectra. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained. Spectroscopy Of Emission.
From www.researchgate.net
Schematic arrangement of direct current arc optical emission spectroscopy. Download Scientific Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns. Spectroscopy Of Emission.
From chemwiki.ucdavis.edu
10G Atomic Emission Spectroscopy Chemwiki Spectroscopy Of Emission 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. spectroscopy is a general methodology that can. Spectroscopy Of Emission.
From www.researchgate.net
THz emission spectroscopy and sample characterization. a) Schematic... Download Scientific Diagram Spectroscopy Of Emission Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. 10.7.1 atomic emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained. Spectroscopy Of Emission.
From users.highland.edu
Atomic Spectra and Models of the Atom Spectroscopy Of Emission 10.7.1 atomic emission spectra. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. the emission spectrum (or line spectrum) of a. Spectroscopy Of Emission.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Spectroscopy Of Emission 10.7.1 atomic emission spectra. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. atomic emission spectroscopy (aes) is a method of. Spectroscopy Of Emission.
From es.slideshare.net
Emission spectroscopy Spectroscopy Of Emission Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected. Spectroscopy Of Emission.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Spectroscopy Of Emission 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. Atomic emission occurs when a valence electron in a higher energy atomic. Spectroscopy Of Emission.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Atomic emission occurs when a valence electron in a higher energy atomic orbital. Spectroscopy Of Emission.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy Of Emission the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. spectroscopy is a general methodology that can be adapted in many. Spectroscopy Of Emission.
From www.mdpi.com
Processes Free FullText Optical Emission Spectroscopy of Underwater Spark Generated by Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have. Spectroscopy Of Emission.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Vector Illustration of light Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained. Spectroscopy Of Emission.
From penrodkur.blogspot.com
Line Spectrum Of Hydrogen Modelo Atómico de Bohr Características y Postulados Lifeder / Our Spectroscopy Of Emission The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. spectroscopy is a general methodology that can be adapted in many ways to extract the information. Spectroscopy Of Emission.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra? HubPages Spectroscopy Of Emission Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with. Spectroscopy Of Emission.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten... Download Scientific Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. . Spectroscopy Of Emission.
From www.youtube.com
2.2 Hydrogen emission spectrum (SL) YouTube Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. spectroscopy is a general methodology that can be adapted in many ways. Spectroscopy Of Emission.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Presentation ID494340 Spectroscopy Of Emission the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. spectroscopy is a general methodology that can be adapted in many. Spectroscopy Of Emission.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/104 Resource Book Spectroscopy Of Emission The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Spectroscopy Of Emission.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Spectroscopy Of Emission Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light. Spectroscopy Of Emission.
From byjus.com
Emission spectrum Atomic spectra Chemistry Spectroscopy Of Emission The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. 10.7.1 atomic emission spectra. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. atomic emission spectroscopy (aes) is a method of. Spectroscopy Of Emission.
From spiff.rit.edu
Spectrographs and Spectra Spectroscopy Of Emission 10.7.1 atomic emission spectra. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. The emission spectrum is the set of light frequencies emitted. Spectroscopy Of Emission.
From www.youtube.com
Flame photometry/Flame Emission Spectroscopy (FES)/Atomic emission spectroscopy (AES) YouTube Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. 10.7.1 atomic emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. atomic emission spectroscopy (aes). Spectroscopy Of Emission.
From microbiologynotes.org
Spectroscopy Introduction, Principles, Types and Applications Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have. Spectroscopy Of Emission.
From www.youtube.com
Types of Spectroscopy Atomic and Molecular Absorption and Emission Spectroscopy Principle Spectroscopy Of Emission the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. 10.7.1 atomic emission spectra. The emission spectrum is the set of light frequencies emitted by substances after. Spectroscopy Of Emission.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. the emission spectrum (or line spectrum) of a chemical element is the unique. Spectroscopy Of Emission.
From stock.adobe.com
Continuous spectrum, an emission spectrum that consists of continuum of wavelengths without any Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. The emission spectrum is the set of light frequencies emitted. Spectroscopy Of Emission.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the. Spectroscopy Of Emission.
From www.researchgate.net
CO 2 optical emission spectroscopy (OES) (a) Schematic of the... Download Scientific Diagram Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. spectroscopy is a general methodology that can be adapted in many ways. Spectroscopy Of Emission.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Spectroscopy Of Emission spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational, rotational. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have. Spectroscopy Of Emission.
From www.researchgate.net
Optical emission spectrum of RF nitrogen plasma and its active species. Download Scientific Spectroscopy Of Emission Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. spectroscopy is a general methodology that can be adapted in many ways to extract the information you need (energies of electronic, vibrational,. Spectroscopy Of Emission.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. The emission spectrum is the set of light frequencies emitted by substances after they have been excited with various forms of energy,. Atomic emission occurs when a valence electron in a higher energy atomic orbital returns to a. . Spectroscopy Of Emission.
From www.priyamstudycentre.com
Spectroscopy Definition, Types, Applications Spectroscopy Of Emission atomic emission spectroscopy (aes) is a method of chemical analysis that uses the intensity of light emitted from a flame,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Atomic emission occurs when a valence electron in a higher energy atomic orbital. Spectroscopy Of Emission.