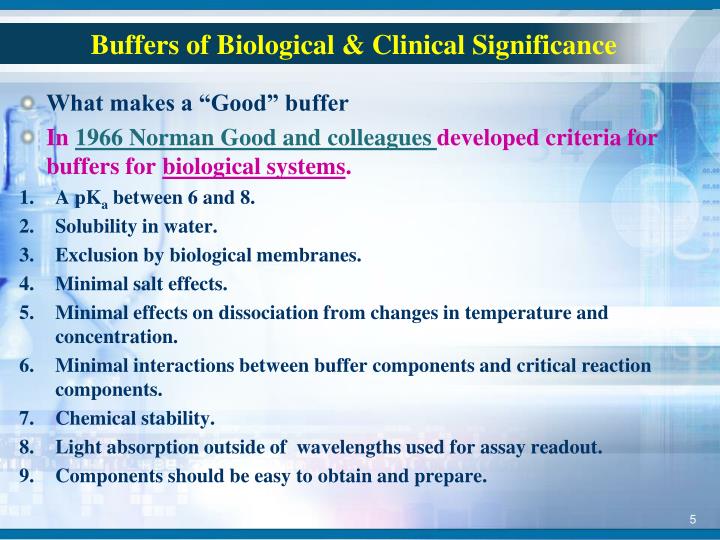
What Makes A Good Buffer . Explore examples of buffers in chemistry and biology,. Has the largest buffer capacity for its concentration). Also, the solubility level of good. A buffer is a solution that can resist ph change upon the addition of an acidic or. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. — the buffer range is the range of ph values over which a buffer is most effective (i.e. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. buffers work well only for limited amounts of added strong acid or base. what is a buffer solution? — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. — introduction to buffers. — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph.
from www.slideserve.com
a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. Explore examples of buffers in chemistry and biology,. buffers work well only for limited amounts of added strong acid or base. what is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Has the largest buffer capacity for its concentration). — introduction to buffers. — the buffer range is the range of ph values over which a buffer is most effective (i.e. — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph.
PPT Buffers of Biological & Clinical Significance PowerPoint
What Makes A Good Buffer — introduction to buffers. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. what is a buffer solution? Has the largest buffer capacity for its concentration). — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. — introduction to buffers. buffers work well only for limited amounts of added strong acid or base. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Explore examples of buffers in chemistry and biology,. — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. — the buffer range is the range of ph values over which a buffer is most effective (i.e. A buffer is a solution that can resist ph change upon the addition of an acidic or. Also, the solubility level of good.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Makes A Good Buffer Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. buffers work well only for limited amounts of added strong acid or base. — introduction to buffers. A buffer is a solution that can resist ph change upon the addition of an acidic or. — the buffer range is the range. What Makes A Good Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Makes A Good Buffer — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. — good buffers have a high solubility in water, since most biological. What Makes A Good Buffer.
From www.talkbass.com
What makes a good buffer, who makes a good buffer and which buffer What Makes A Good Buffer — the buffer range is the range of ph values over which a buffer is most effective (i.e. — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. A buffer is a solution that can resist ph change upon the addition of an acidic or. — learn what. What Makes A Good Buffer.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Makes A Good Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. Also, the solubility level of good. — the buffer range is the range of ph values over which a buffer. What Makes A Good Buffer.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Makes A Good Buffer Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. — the buffer range is the range of ph values over which a buffer is most effective (i.e. Also, the solubility level of good. buffers work well only for limited amounts of added strong acid or base. Has the largest buffer capacity. What Makes A Good Buffer.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid What Makes A Good Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Explore examples of buffers in chemistry and biology,. — introduction to buffers. what is a buffer solution? — learn what a buffer is, how it works, and how to choose the best buffer for. What Makes A Good Buffer.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your What Makes A Good Buffer a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Has the largest buffer capacity for its concentration). Once either solute. What Makes A Good Buffer.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Makes A Good Buffer — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. buffers work well only for limited amounts of added strong acid or base. — introduction to buffers. what is a buffer solution? a buffer solution is a solution where the ph does not change significantly on. What Makes A Good Buffer.
From studylib.net
The SOLUTION for All of Your Buffer Needs What Makes A Good Buffer Has the largest buffer capacity for its concentration). a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. what is a buffer solution? — the buffer range is the range of ph values over which a buffer is most effective (i.e. . What Makes A Good Buffer.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Makes A Good Buffer Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. Explore examples of buffers in chemistry and biology,. Also, the solubility level of good. — introduction to buffers. buffers work well only for limited amounts of added strong acid or base. what is a buffer solution? — learn what a. What Makes A Good Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Makes A Good Buffer — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. buffers work well only for limited amounts of added strong acid or base. — the buffer range. What Makes A Good Buffer.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Makes A Good Buffer — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Explore examples of buffers in chemistry and biology,. Once either solute is all reacted, the solution is. What Makes A Good Buffer.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks What Makes A Good Buffer buffers work well only for limited amounts of added strong acid or base. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer is a solution that can resist ph change upon the addition of an acidic or. Has the largest buffer capacity for. What Makes A Good Buffer.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Makes A Good Buffer — introduction to buffers. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Explore examples of buffers in chemistry and biology,. Has the largest buffer capacity for its concentration). — learn what a buffer is, how it works, and how to choose the best. What Makes A Good Buffer.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint What Makes A Good Buffer Also, the solubility level of good. buffers work well only for limited amounts of added strong acid or base. what is a buffer solution? — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. Has the largest buffer capacity for its concentration). A buffer is a solution. What Makes A Good Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Makes A Good Buffer what is a buffer solution? a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. A buffer is a solution that can resist. What Makes A Good Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Makes A Good Buffer — introduction to buffers. Also, the solubility level of good. — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. what is a buffer solution? — the buffer range is the range of ph values over which a buffer is most effective (i.e. Has the largest. What Makes A Good Buffer.
From www.expii.com
Buffers — Definition & Overview Expii What Makes A Good Buffer — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. Also, the solubility level of good. — the buffer range is the range of ph values over which a buffer is most effective. What Makes A Good Buffer.
From www.chemicals.co.uk
What Makes A Good Buffer In Chemistry? The Chemistry Blog What Makes A Good Buffer — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. . What Makes A Good Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium What Makes A Good Buffer a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. Explore examples of buffers in chemistry and biology,. what is a buffer solution?. What Makes A Good Buffer.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID1149749 What Makes A Good Buffer Has the largest buffer capacity for its concentration). A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. Explore examples of buffers in chemistry and biology,.. What Makes A Good Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Makes A Good Buffer what is a buffer solution? buffers work well only for limited amounts of added strong acid or base. Has the largest buffer capacity for its concentration). Also, the solubility level of good. A buffer is a solution that can resist ph change upon the addition of an acidic or. — good buffers have a high solubility in. What Makes A Good Buffer.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Makes A Good Buffer — introduction to buffers. what is a buffer solution? — the buffer range is the range of ph values over which a buffer is most effective (i.e. — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. A buffer solution is one which resists changes in ph. What Makes A Good Buffer.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your What Makes A Good Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Explore examples of buffers in chemistry and biology,. A buffer is a solution that can resist ph change upon the addition of an acidic or. what is a buffer solution? buffers work well only for. What Makes A Good Buffer.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Makes A Good Buffer what is a buffer solution? — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. A buffer is a solution that can resist ph change upon the addition of an acidic or.. What Makes A Good Buffer.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide What Makes A Good Buffer — the buffer range is the range of ph values over which a buffer is most effective (i.e. A buffer is a solution that can resist ph change upon the addition of an acidic or. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. a buffer solution is a solution where. What Makes A Good Buffer.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Makes A Good Buffer — introduction to buffers. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Also, the solubility level of good. Has the largest buffer capacity for its concentration). —. What Makes A Good Buffer.
From sciencing.com
Characteristics of Good Buffers Sciencing What Makes A Good Buffer — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. A buffer is a solution that can resist ph change upon the addition of an acidic or. — introduction to buffers. A buffer solution is one which resists changes in ph when small quantities of an acid or. What Makes A Good Buffer.
From www.youtube.com
What makes a good buffer? YouTube What Makes A Good Buffer Has the largest buffer capacity for its concentration). Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. A buffer solution is one which resists changes in ph when small quantities of an acid. What Makes A Good Buffer.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Makes A Good Buffer Also, the solubility level of good. buffers work well only for limited amounts of added strong acid or base. Has the largest buffer capacity for its concentration). — introduction to buffers. Explore examples of buffers in chemistry and biology,. a buffer solution is a solution where the ph does not change significantly on dilution or if an. What Makes A Good Buffer.
From slidetodoc.com
PREPARATION OF BUFFERS To make an effective buffer What Makes A Good Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. — introduction to buffers. Also, the solubility level of good. buffers work well only for limited amounts of added. What Makes A Good Buffer.
From publicaffairsworld.com
what are buffers and why are they important to life What Makes A Good Buffer Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. Explore examples of buffers in chemistry and biology,. Has the largest buffer capacity for its concentration). — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. a buffer solution is a solution where. What Makes A Good Buffer.
From www.youtube.com
What makes a good buffer? YouTube What Makes A Good Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. what is a buffer solution? — good buffers have a high solubility in water, since most biological systems naturally use water as their solvent. — introduction to buffers. a buffer solution is a. What Makes A Good Buffer.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Makes A Good Buffer — introduction to buffers. buffers work well only for limited amounts of added strong acid or base. — learn what a buffer is, how it works, and how to choose the best buffer for a desired ph. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid. What Makes A Good Buffer.
From www.promegaconnections.com
What Makes a "Good" Buffer? What Makes A Good Buffer buffers work well only for limited amounts of added strong acid or base. — introduction to buffers. — the buffer range is the range of ph values over which a buffer is most effective (i.e. Also, the solubility level of good. Has the largest buffer capacity for its concentration). what is a buffer solution? —. What Makes A Good Buffer.