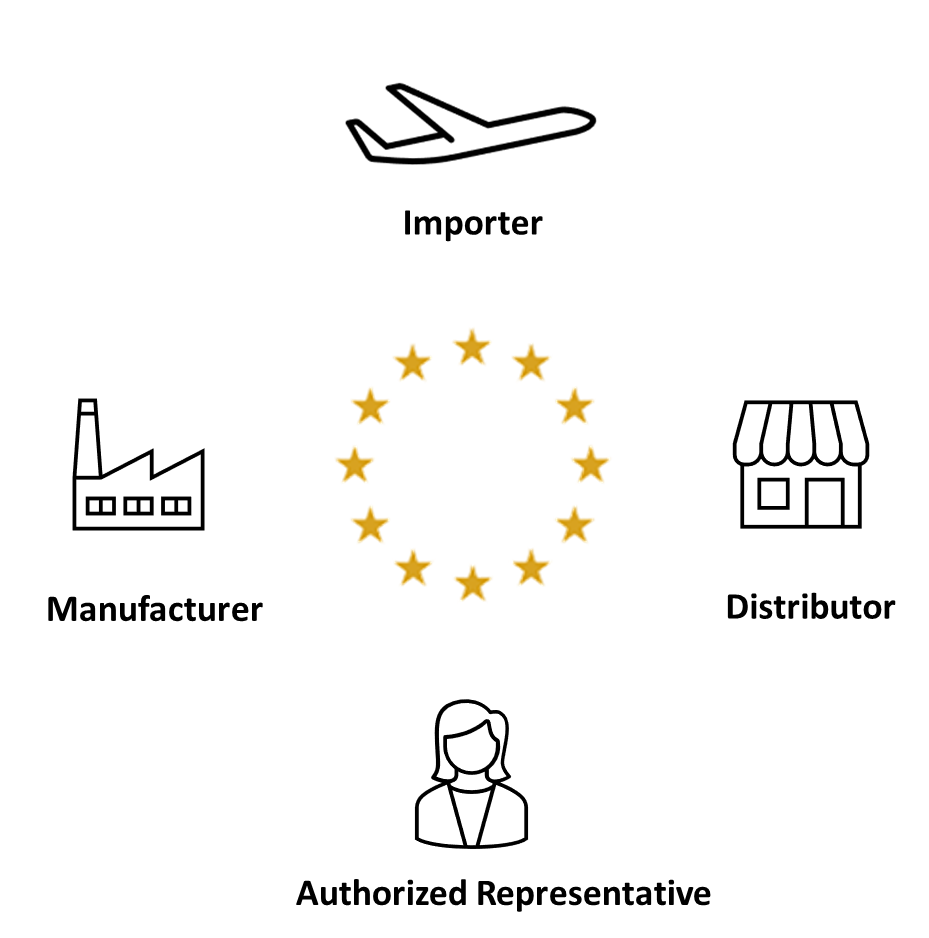
Eu Mdr Distributor Labeling Requirements . there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Increased focus on clarity and on. the regulations clarify the respective responsibilities of authorised representatives, importers and. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. general requirements (23.1) performance information to be in labelling. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu).
from www.qualitiso.com
Increased focus on clarity and on. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the regulations clarify the respective responsibilities of authorised representatives, importers and. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. general requirements (23.1) performance information to be in labelling. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the.
Roles and obligations in the Medical Devices Regulation
Eu Mdr Distributor Labeling Requirements general requirements (23.1) performance information to be in labelling. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the regulations clarify the respective responsibilities of authorised representatives, importers and. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. Increased focus on clarity and on. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). general requirements (23.1) performance information to be in labelling. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.
From www.regulatoryglobe.com
EU MDR implementation guide for medical devices MDCG Eu Mdr Distributor Labeling Requirements the regulations clarify the respective responsibilities of authorised representatives, importers and. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). Increased focus on clarity and on. general requirements (23.1) performance information. Eu Mdr Distributor Labeling Requirements.
From www.greenlight.guru
EU MDR and IVDR Distributor Requirements [Overview] Eu Mdr Distributor Labeling Requirements general requirements (23.1) performance information to be in labelling. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the regulations clarify the respective responsibilities of authorised representatives, importers and. regulation (eu) 2017/745 of the european parliament and of the. Eu Mdr Distributor Labeling Requirements.
From www.greenlight.guru
EU MDR and IVDR Distributor Requirements [Overview] Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. general requirements (23.1) performance information to be in. Eu Mdr Distributor Labeling Requirements.
From lifesciences.csoftintl.com
The EU MDR Labeling Journey Best Practices for Navigating the Latest Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. regulation (eu) 2017/745. Eu Mdr Distributor Labeling Requirements.
From www.greenlight.guru
EU MDR Implementation Guide Eu Mdr Distributor Labeling Requirements this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). Increased focus on clarity and on. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. general requirements (23.1) performance information to be in labelling. the regulations clarify the. Eu Mdr Distributor Labeling Requirements.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Eu Mdr Distributor Labeling Requirements this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. general requirements (23.1) performance information to be in labelling. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that. Eu Mdr Distributor Labeling Requirements.
From spyro-soft.com
The Complete Guide to EU Medical Device Regulation Spyrosoft Eu Mdr Distributor Labeling Requirements this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Increased focus on clarity and on. general requirements (23.1) performance information to be in labelling. distributors should verify that the devices have. Eu Mdr Distributor Labeling Requirements.
From www.meddeviceonline.com
8 Key Changes To Understand In The New European MDR And IVDR Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. the regulations clarify the respective responsibilities of authorised representatives, importers and. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds. Eu Mdr Distributor Labeling Requirements.
From www.trinzo.com
MDR Labelling Requirements in the EU What Manufactures Need to Know Eu Mdr Distributor Labeling Requirements the regulations clarify the respective responsibilities of authorised representatives, importers and. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. distributors should verify that the devices have been. Eu Mdr Distributor Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Eu Mdr Distributor Labeling Requirements this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. the regulations clarify the respective responsibilities of authorised representatives, importers and. regulation (eu) 2017/745 of the european parliament and of the council. Eu Mdr Distributor Labeling Requirements.
From www.qualitiso.com
Roles and obligations in the Medical Devices Regulation Eu Mdr Distributor Labeling Requirements regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the regulations clarify the respective responsibilities of authorised representatives, importers and. Increased focus on clarity and on. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. this document. Eu Mdr Distributor Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Eu Mdr Distributor Labeling Requirements distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. the regulations clarify the respective responsibilities of authorised representatives, importers and. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. this document presents. Eu Mdr Distributor Labeling Requirements.
From www.youtube.com
Introduction to Medical Device Labeling Symbols YouTube Eu Mdr Distributor Labeling Requirements regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the regulations clarify the respective responsibilities of authorised representatives, importers and.. Eu Mdr Distributor Labeling Requirements.
From www.greenlight.guru
Ultimate Guide to Device Class Requirements under EU MDR Eu Mdr Distributor Labeling Requirements regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). general requirements (23.1) performance information to be in labelling. Increased focus on clarity and on. there are a number. Eu Mdr Distributor Labeling Requirements.
From www.rimsys.io
The ultimate guide to the EU MDR and IVDR general safety and Eu Mdr Distributor Labeling Requirements there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. general requirements (23.1) performance information to be in labelling. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that.. Eu Mdr Distributor Labeling Requirements.
From www.freyrsolutions.com
EU MDR vs. EU IVDR Key Labeling and Artwork Variations Freyr Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for. Eu Mdr Distributor Labeling Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Eu Mdr Distributor Labeling Requirements general requirements (23.1) performance information to be in labelling. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. the regulations clarify the respective responsibilities of authorised representatives, importers and. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). Increased focus. Eu Mdr Distributor Labeling Requirements.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). regulation (eu) 2017/745 of the european parliament and. Eu Mdr Distributor Labeling Requirements.
From rookqs.com
Best Practices For a SaMD MDR Application Rook Quality Systems Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the regulations clarify the respective responsibilities of authorised representatives, importers and. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds. Eu Mdr Distributor Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. the regulations clarify the respective responsibilities of authorised representatives, importers and. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds. Eu Mdr Distributor Labeling Requirements.
From www.johner-institute.com
MDR Distributor Requirements (that also affect the manufacturers) Eu Mdr Distributor Labeling Requirements there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. the regulations clarify the respective responsibilities of authorised representatives, importers and. Increased focus on clarity and on. distributors should verify that the devices have been ce marked, that an eu declaration. Eu Mdr Distributor Labeling Requirements.
From www.rqmplus.com
Solving the EU MDR Labeling Puzzle Eu Mdr Distributor Labeling Requirements general requirements (23.1) performance information to be in labelling. Increased focus on clarity and on. the regulations clarify the respective responsibilities of authorised representatives, importers and. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). regulation (eu) 2017/745 of the european parliament and of the council of 5. Eu Mdr Distributor Labeling Requirements.
From medicaldevices.freyrsolutions.com
EU MDR Compliance Services, EU MDR Requirements Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. the regulations clarify the respective responsibilities of authorised representatives, importers and. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. . Eu Mdr Distributor Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Eu Mdr Distributor Labeling Requirements general requirements (23.1) performance information to be in labelling. the regulations clarify the respective responsibilities of authorised representatives, importers and. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn. Eu Mdr Distributor Labeling Requirements.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Eu Mdr Distributor Labeling Requirements distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. there are a number of new distributor requirements in eu mdr and eu ivdr, and. Eu Mdr Distributor Labeling Requirements.
From www.artixio.com
Navigating the EU MDR Labelling Requirements A Comprehensive Guide Eu Mdr Distributor Labeling Requirements general requirements (23.1) performance information to be in labelling. the regulations clarify the respective responsibilities of authorised representatives, importers and. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m. Eu Mdr Distributor Labeling Requirements.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Eu Mdr Distributor Labeling Requirements general requirements (23.1) performance information to be in labelling. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. the regulations clarify the respective responsibilities of authorised representatives, importers and. Increased focus on clarity and on. there are a number of new distributor requirements. Eu Mdr Distributor Labeling Requirements.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Eu Mdr Distributor Labeling Requirements regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the regulations clarify the respective responsibilities of authorised representatives, importers and. general requirements (23.1) performance information to be in labelling. there are a number of new distributor requirements in eu mdr and eu ivdr, and in. Eu Mdr Distributor Labeling Requirements.
From mavig.com
New Product Labeling due to MDR MAVIG Eu Mdr Distributor Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. distributors should verify that the devices have been ce marked, that an eu declaration. Eu Mdr Distributor Labeling Requirements.
From www.youtube.com
EU MDR Labeling Compliance Learn the Lessons from UDI YouTube Eu Mdr Distributor Labeling Requirements Increased focus on clarity and on. the regulations clarify the respective responsibilities of authorised representatives, importers and. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. general requirements (23.1) performance information to be in labelling. there are a number of new distributor requirements in eu. Eu Mdr Distributor Labeling Requirements.
From www.studocu.com
New Labeling Requirement as EU MDR 745 2017 Labelling General Eu Mdr Distributor Labeling Requirements distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. regulation (eu) 2017/745 of the european parliament and of. Eu Mdr Distributor Labeling Requirements.
From www.meddevo.com
meddevo Blog MDR Labeling Explained! Eu Mdr Distributor Labeling Requirements there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). distributors should verify that the devices have been ce marked, that an eu declaration. Eu Mdr Distributor Labeling Requirements.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Eu Mdr Distributor Labeling Requirements the regulations clarify the respective responsibilities of authorised representatives, importers and. general requirements (23.1) performance information to be in labelling. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. this document presents questions and answers about obligations introduced by article 16(2) to (4). Eu Mdr Distributor Labeling Requirements.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Eu Mdr Distributor Labeling Requirements there are a number of new distributor requirements in eu mdr and eu ivdr, and in this article, i’m going to explain those requirements and the. this document presents questions and answers about obligations introduced by article 16(2) to (4) under regulation (eu). general requirements (23.1) performance information to be in labelling. regulation (eu) 2017/745 of. Eu Mdr Distributor Labeling Requirements.
From www.youtube.com
How to be EU MDR Labeling Compliance Ready by Using Lessons from UDI Eu Mdr Distributor Labeling Requirements the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. the regulations clarify the respective responsibilities of authorised representatives, importers and. Increased focus on clarity and on. distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that. . Eu Mdr Distributor Labeling Requirements.