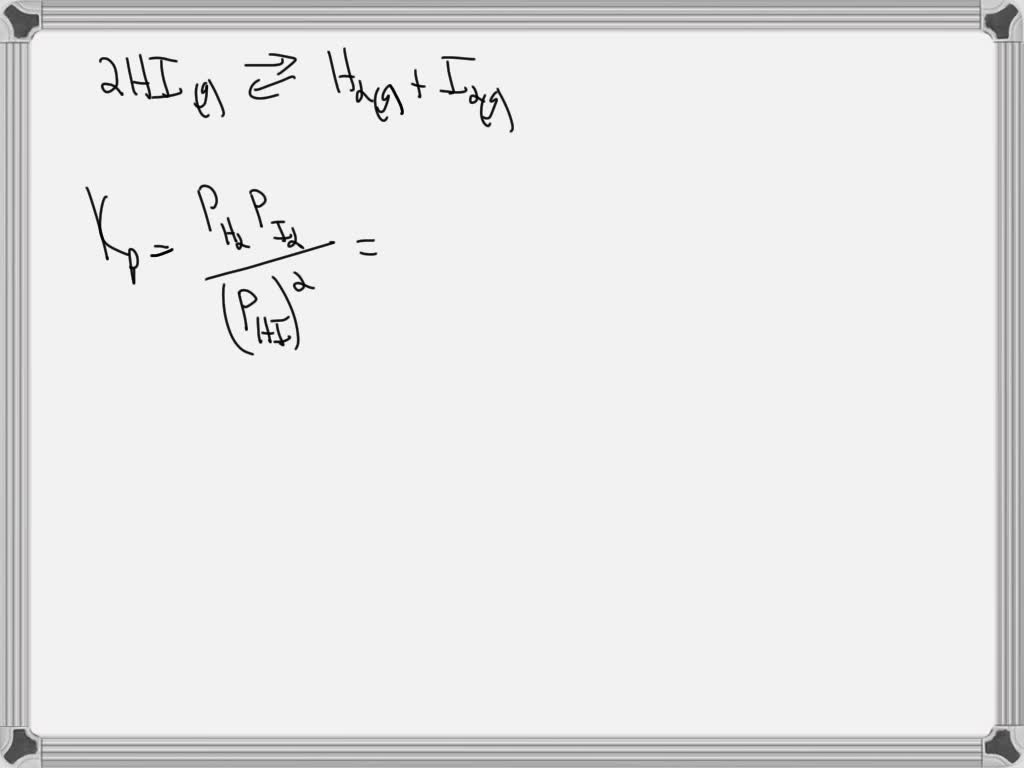Reaction Vessel Is Increased . this process is described by le châtelier’s principle: The condition in a reaction when the concentrations of reactants and products cease to change. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. ratef = rater rate f = rate r. learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. Describe the conditions for successful collisions that cause reactions. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. change the pressure: Describe rate in terms of.
from www.numerade.com
Describe the conditions for successful collisions that cause reactions. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. this process is described by le châtelier’s principle: The condition in a reaction when the concentrations of reactants and products cease to change. learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. change the pressure: Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. Describe rate in terms of. ratef = rater rate f = rate r.
SOLVED A reaction vessel is charged with hydrogen iodide, which
Reaction Vessel Is Increased Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. The condition in a reaction when the concentrations of reactants and products cease to change. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. ratef = rater rate f = rate r. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. Describe the conditions for successful collisions that cause reactions. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. Describe rate in terms of. learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. this process is described by le châtelier’s principle: change the pressure:
From www.researchgate.net
Reaction vessel for microsponge preparation by liquidliquid suspension Reaction Vessel Is Increased Describe the conditions for successful collisions that cause reactions. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. change the pressure: learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. If the system is stressed by adding reactant, either. Reaction Vessel Is Increased.
From www.researchgate.net
Reaction vessel image after completion of the reaction Download Reaction Vessel Is Increased ratef = rater rate f = rate r. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. Describe rate in terms of. learn about le chatelier's principle and. Reaction Vessel Is Increased.
From www.slideserve.com
PPT Industrial Chemistry PowerPoint Presentation, free download ID Reaction Vessel Is Increased The condition in a reaction when the concentrations of reactants and products cease to change. learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. . Reaction Vessel Is Increased.
From www.numerade.com
SOLVED A reaction vessel is charged with hydrogen iodide, which Reaction Vessel Is Increased If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. change the pressure: Describe the conditions for successful collisions that cause reactions. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. Describe rate. Reaction Vessel Is Increased.
From www.researchgate.net
Purging efficiency of mercury species from the reaction vessel Reaction Vessel Is Increased Describe the conditions for successful collisions that cause reactions. Describe rate in terms of. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. change the pressure: When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. The condition in a reaction when the concentrations. Reaction Vessel Is Increased.
From www.bartleby.com
Answered A reaction vessel initially contains… bartleby Reaction Vessel Is Increased If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. Describe rate in terms of. Describe the conditions for successful collisions that cause reactions. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. learn. Reaction Vessel Is Increased.
From www.researchgate.net
TOP VIEW OF THE REACTION VESSEL WITH PRESSURE TRANSDUCERS POSITIONING Reaction Vessel Is Increased When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. change the pressure: ratef = rater rate f = rate r. Describe rate in terms of. The condition in a reaction when the. Reaction Vessel Is Increased.
From www.youtube.com
Acute Inflammation, Vascular Permeability & Vasodilation Fluid Phase Reaction Vessel Is Increased The condition in a reaction when the concentrations of reactants and products cease to change. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. ratef = rater rate f = rate r. learn about le chatelier's principle and how changing volume affects. Reaction Vessel Is Increased.
From reactionvessel.co.in
Reaction Vessel, Chemical Reaction Vessel, Stainless Steel Reaction Reaction Vessel Is Increased in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. The condition in a reaction when the concentrations of reactants and products cease to change. When a chemical system at equilibrium is disturbed, it returns to. Reaction Vessel Is Increased.
From reactor.co.in
Reaction Vessel Reaction Vessel Is Increased this process is described by le châtelier’s principle: change the pressure: The condition in a reaction when the concentrations of reactants and products cease to change. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. Describe rate in terms of. learn. Reaction Vessel Is Increased.
From www.researchgate.net
Schematic of reaction vessel (A) and membrane inlet mass spectrometer Reaction Vessel Is Increased according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. ratef = rater rate f = rate r. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. Describe the conditions for successful collisions that cause reactions. The condition in. Reaction Vessel Is Increased.
From www.researchgate.net
Purging efficiency of mercury species from the reaction vessel Reaction Vessel Is Increased learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. Describe the conditions for successful collisions that cause reactions. change the pressure: If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. according to. Reaction Vessel Is Increased.
From www.numerade.com
SOLVEDIf the pressure in a reaction vessel for the following reaction Reaction Vessel Is Increased change the pressure: If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. in other words, if there is an increase in products, the. Reaction Vessel Is Increased.
From www.numerade.com
Let Analyze Identily what factor iS descnbed Compressing two gasses in Reaction Vessel Is Increased ratef = rater rate f = rate r. The condition in a reaction when the concentrations of reactants and products cease to change. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. this process is described by le châtelier’s principle: When a. Reaction Vessel Is Increased.
From ribbonblendermixer.com
Reaction Vessels, Reaction Kettle, Chemical Reaction Vessels Reaction Vessel Is Increased When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. Describe rate in terms of. ratef = rater rate f = rate r. Increasing the volume of the reaction vessel will cause a decrease. Reaction Vessel Is Increased.
From www.chegg.com
Solved For the reaction of CO_2 (g) + NO(g) rightarrow Reaction Vessel Is Increased Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. Describe rate in terms of. Describe the conditions for successful collisions that cause reactions. When a chemical system at equilibrium is disturbed, it returns to equilibrium. Reaction Vessel Is Increased.
From amglassware.com
Jacketed Reaction Vessels And Reactors Constructed From Borosilicate Glass Reaction Vessel Is Increased Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. change the pressure: If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. Describe the conditions for successful collisions that cause reactions. learn about le chatelier's principle and how. Reaction Vessel Is Increased.
From www.researchgate.net
Scheme of the reaction vessel for CO 2 desorption with a builtin Reaction Vessel Is Increased Describe rate in terms of. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. this process is described by le châtelier’s principle: change the pressure: The condition in a reaction when the concentrations of reactants and products cease to change. learn about le chatelier's principle and how. Reaction Vessel Is Increased.
From www.numerade.com
SOLVED An evacuated reaction vessel is filled with 0.800 atm of N₂ and Reaction Vessel Is Increased If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. Describe rate in terms of. The condition in a reaction when the concentrations of reactants and products cease to change. Describe the conditions for successful collisions that cause reactions. change the pressure: When a. Reaction Vessel Is Increased.
From amglassware.com
Reaction Vessels for laboratory applications with thermostatic jackets Reaction Vessel Is Increased this process is described by le châtelier’s principle: If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. Describe the conditions for successful collisions that. Reaction Vessel Is Increased.
From www.numerade.com
A reaction vessel for synthesizing ammonia by reacting nitrogen and Reaction Vessel Is Increased learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. change the pressure: Describe the conditions for successful collisions that cause reactions. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. Describe rate in. Reaction Vessel Is Increased.
From www.researchgate.net
Internal reaction vessel temperature according to heating time (2.5, 5 Reaction Vessel Is Increased When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. Describe rate in terms of. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. in other words, if there is an increase in products, the reaction quotient, \(q_c\),. Reaction Vessel Is Increased.
From www.researchgate.net
Effect of reaction vessel surface and shear on enzymatic hydrolysis of Reaction Vessel Is Increased learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. change the pressure: ratef = rater rate f = rate r. The condition in a reaction when the concentrations of reactants and products cease to. Reaction Vessel Is Increased.
From www.youtube.com
13.8 g of N2O4 was placed in a 1 L reaction vessel at 400 K and allowed Reaction Vessel Is Increased learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. this process is described by le châtelier’s principle: Describe the conditions for successful collisions that cause reactions. The condition in a reaction when the concentrations of reactants and products cease to change. change the pressure: Describe rate in terms. Reaction Vessel Is Increased.
From www.numerade.com
SOLVED12.A reaction vessel initially contains six molecules of Nz and Reaction Vessel Is Increased If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. ratef = rater rate f = rate r. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. Increasing the volume of the reaction vessel will cause a decrease. Reaction Vessel Is Increased.
From philschatz.com
Hemostasis · Anatomy and Physiology Reaction Vessel Is Increased in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. The condition in a reaction when the concentrations of reactants and products cease to change. Describe rate in terms of. . Reaction Vessel Is Increased.
From www.researchgate.net
7 Reaction vessel for online HPLC µextraction experiments. The size Reaction Vessel Is Increased according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. When a chemical system at equilibrium is. Reaction Vessel Is Increased.
From www.asynt.com
Laboratory Reaction Vessels Asynt Reaction Vessel Is Increased this process is described by le châtelier’s principle: in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. When a chemical system at equilibrium is disturbed, it returns to equilibrium by counteracting the. learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's. Reaction Vessel Is Increased.
From www.manufacturingchemist.com
Improved reaction vessel switching in flow chemistry Reaction Vessel Is Increased learn about le chatelier's principle and how changing volume affects chemical equilibrium with khan academy's ap chemistry course. this process is described by le châtelier’s principle: ratef = rater rate f = rate r. change the pressure: Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. The condition in a reaction. Reaction Vessel Is Increased.
From www.researchgate.net
13. Experiment reaction vessel and components. Download Scientific Reaction Vessel Is Increased according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. If the system is stressed by adding reactant, either h 2 or i 2, the resulting increase in concentration causes the rate of the forward. ratef = rater rate f = rate r. this process is described by le. Reaction Vessel Is Increased.
From pixels.com
Iter Reaction Vessel Photograph by David Parker Pixels Reaction Vessel Is Increased The condition in a reaction when the concentrations of reactants and products cease to change. Describe rate in terms of. this process is described by le châtelier’s principle: in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. according to le chatelier's principle, if pressure is increased, then. Reaction Vessel Is Increased.
From www.numerade.com
SOLVEDThe volume of the reaction vessel containing an equilibrium Reaction Vessel Is Increased according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. ratef = rater rate f = rate r. If the system is stressed by adding reactant, either h 2 or. Reaction Vessel Is Increased.
From www.researchgate.net
Schematic diagrams of the reaction vessel and auxiliary instruments Reaction Vessel Is Increased The condition in a reaction when the concentrations of reactants and products cease to change. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. ratef = rater rate f = rate r. Describe the conditions. Reaction Vessel Is Increased.
From www.researchgate.net
4. Schematic of Reaction Vessel [The diagram shows solid and liquid Reaction Vessel Is Increased according to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the. Describe the conditions for successful collisions that cause reactions. Increasing the volume of the reaction vessel will cause a decrease in partial pressures,. this process is described by le châtelier’s principle: When a chemical system at equilibrium is disturbed, it. Reaction Vessel Is Increased.
From www.doubtnut.com
The volume of the reaction vessel containing an equilibrium mixture is Reaction Vessel Is Increased this process is described by le châtelier’s principle: Describe the conditions for successful collisions that cause reactions. in other words, if there is an increase in products, the reaction quotient, \(q_c\), is increased, making it greater. ratef = rater rate f = rate r. change the pressure: Increasing the volume of the reaction vessel will cause. Reaction Vessel Is Increased.