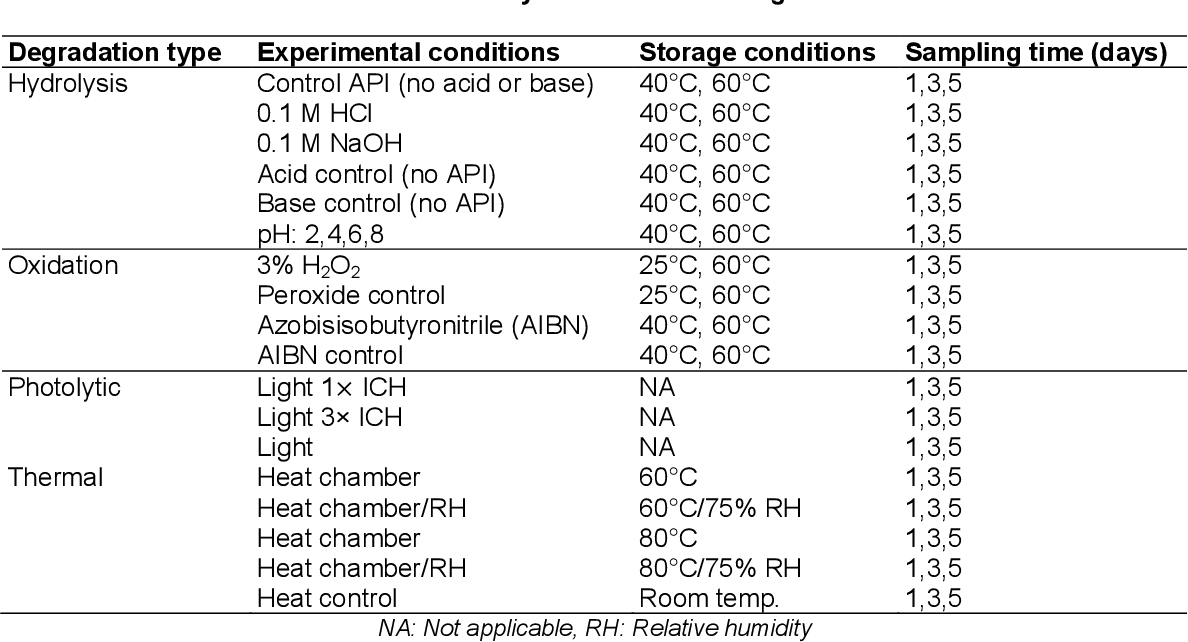
Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of . Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. However, product maturation may necessitate shelf life extension, requiring new stability studies. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Early detection and corrective action can lead to a drug. In vitro dissolution testing for solid dosage forms. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters.
from www.semanticscholar.org
Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. In vitro dissolution testing for solid dosage forms. However, product maturation may necessitate shelf life extension, requiring new stability studies. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. Early detection and corrective action can lead to a drug. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood.
Table 4 from An Approach to Drug Stability Studies and Shelflife Determination Semantic Scholar
Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of In vitro dissolution testing for solid dosage forms. In vitro dissolution testing for solid dosage forms. However, product maturation may necessitate shelf life extension, requiring new stability studies. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. Early detection and corrective action can lead to a drug. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava.
From www.slideserve.com
PPT Modeling Approaches to Multiple Isothermal Stability Studies for Estimating Shelf Life Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of In vitro dissolution testing for solid dosage forms. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Early detection and corrective action can lead to a drug. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. However, product maturation may. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.pharmacalculation.com
Shelf Life Calculation of Drug Product Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. However, product maturation may necessitate shelf. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From studylib.net
Guidance note 18 Determination of Product Shelf Life Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. In vitro dissolution testing for solid dosage forms. Stability is determined by evaluation of the quality parameters with time under the influence of a. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.researchgate.net
(PDF) AN OVERVIEW STABILITY STUDY OF PHARMACEUTICAL PRODUCTS AND SHELF LIFE PREDICTION Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of In vitro dissolution testing for solid dosage forms. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. The duration during which a drug maintains at least 90% of its. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From present5.com
Dosage Form Design Pharmaceutical and Formulation Considerations Chapter Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. However, product maturation. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.youtube.com
Determination of shelf life (I Introduction) Lesson 02 YouTube Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. However, product maturation may necessitate shelf life extension, requiring new stability studies. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Stability is determined by evaluation of the quality parameters with time. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.scribd.com
Shelf Life Determination Based on t90 Values Shelf Life Confidence Interval Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of In vitro dissolution testing for solid dosage forms. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability data can be used to monitor unintended drift in the manufacturing process, according. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.pharmaguideline.com
Shelf Life Estimation of Pharmaceutical Products Pharmaceutical Guidelines Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. However, product maturation may necessitate shelf life extension, requiring new stability studies. In vitro dissolution testing for solid dosage forms. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. Stability is determined. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.slideshare.net
Examining Simplified Shelf Life Testing Methods Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of In vitro dissolution testing for solid dosage forms. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. However, product maturation may necessitate shelf life extension, requiring new stability studies. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. Stability data. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.scribd.com
Drug Shelf Life PDF Shelf Life Pharmaceutical Formulation Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. In vitro dissolution testing for solid dosage forms. The duration during which a drug maintains at least 90% of its labeled potency under specified. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.slideserve.com
PPT Dosage Form Design Pharmaceutical and Formulation Considerations PowerPoint Presentation Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. In vitro dissolution testing for solid dosage forms. Early detection and corrective action can lead to a drug. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. The duration during which. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From mavink.com
Product Shelf Life Chart Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Stability data can be used. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.mdpi.com
Pharmaceutics Free FullText Drug Shelf Life and Release Limits Estimation Based on Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Early detection and corrective action can lead to a drug. In vitro dissolution testing for solid dosage forms. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. However, product maturation may necessitate shelf life extension, requiring new stability studies. Stability is determined by evaluation of the quality parameters with. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.slideserve.com
PPT Lab 6 PowerPoint Presentation, free download ID2351923 Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. In vitro dissolution testing for solid dosage forms. However, product maturation may necessitate shelf life extension, requiring new stability studies. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. Early detection and corrective action can. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.slideserve.com
PPT Dosage Form Design PowerPoint Presentation, free download ID5640672 Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. Early detection and corrective action can. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.scribd.com
Expt. 6 Shelf Life Determination (PhyPhar) Chemical Shelf Life Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of However, product maturation may necessitate shelf life extension, requiring new stability studies. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. In vitro dissolution testing for solid dosage forms. Stability data can be used. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.researchgate.net
(PDF) Guidelines on Stability Studies of Pharmaceutical Products and Shelf Life Estimation Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. In vitro dissolution testing for solid dosage forms. However, product maturation may necessitate shelf life extension, requiring new stability studies. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters.. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.researchgate.net
(PDF) An Approach to Drug Stability Studies and Shelflife Determination Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. In vitro dissolution testing for solid dosage forms. Early detection and corrective action can lead to a drug. However, product maturation may necessitate shelf life extension, requiring. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.slideserve.com
PPT Liquid dosage forms PowerPoint Presentation, free download ID4329709 Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. In vitro dissolution testing for solid dosage forms. Stability data can be used to monitor unintended drift in the manufacturing process, according to. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.numerade.com
SOLVED Question No 23 Multiple Choice Selecl out ol 4 optlons, lor Ihe question belon Shelf Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Early detection and corrective action can lead to a drug. However, product maturation may necessitate shelf life extension, requiring new stability studies. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Stability. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From pharmastate.academy
SOP For Determination of Shelf Life of Solution in Laboratory Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. However, product. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From medicaldeviceacademy.com
Shelf Life Testing Protocol Medical Device Academy Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of However, product maturation may necessitate shelf life extension, requiring new stability studies. In vitro dissolution testing for solid dosage forms. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Early detection and corrective action can lead to a drug. Stability is determined by evaluation of the quality parameters with. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.researchgate.net
Determination of the studied drugs in pharmaceutical dosage forms Download Table Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. In vitro dissolution testing for solid dosage forms. However, product maturation may necessitate shelf life extension, requiring new stability studies. Early detection and corrective action can lead. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From calculator.academy
Shelf Life Calculator Calculator Academy Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. In vitro dissolution testing for solid dosage forms. Early detection and corrective action can lead to a drug. Methods for the preparation. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.semanticscholar.org
Table 4 from An Approach to Drug Stability Studies and Shelflife Determination Semantic Scholar Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. However, product maturation may necessitate shelf life extension, requiring new stability studies. In vitro dissolution testing for solid dosage forms. The duration during which a drug. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.scribd.com
Guidelines on Shelf Life of Some Common Medicines Shelf Life Drugs Free 30day Trial Scribd Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. In vitro dissolution testing. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From roxannalancaster.blogspot.com
shelf life calculator for pharmaceutical products Roxanna Lancaster Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of In vitro dissolution testing for solid dosage forms. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. However, product maturation may necessitate shelf life extension, requiring new stability studies. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. It is not applicable. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.aplyon.com
Shelf Life Procedure Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Early detection and corrective action can lead to a drug. However, product maturation may necessitate shelf life extension, requiring new stability studies. It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. The duration. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.pharmacalculation.com
Shelf Life Calculation of Drug Product Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. Early detection and corrective action can lead to a drug. In vitro dissolution testing for solid dosage forms. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. However, product maturation may necessitate shelf life extension,. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Early detection and corrective action can lead to a drug. However, product maturation may necessitate shelf life extension, requiring new stability studies. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. The duration. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.slideserve.com
PPT Dosage Form Design PowerPoint Presentation ID5640672 Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. Stability data can be used to monitor unintended drift in the manufacturing process, according to srivastava. The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. It is not applicable to biological medicinal products such. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.scribd.com
noteguidancestartshelflifefinisheddosageformannexnoteguidancemanufacturefinished Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Methods for the preparation of specifi c types of dosage forms and drug delivery systems are described in subsequent chapters. However, product maturation may necessitate shelf life extension, requiring new stability studies. Stability is determined by evaluation of the quality parameters with time. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.scribd.com
Shelf Life Determination Nonsteroidal Anti Inflammatory Drug Aspirin Free 30day Trial Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of It is not applicable to biological medicinal products such as vaccines, sera, toxins and allergens, products derived from human blood. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. However, product maturation may necessitate shelf life extension, requiring new stability studies. Methods for the preparation of specifi c types of dosage. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From hxerayeok.blob.core.windows.net
Stability Testing And Shelf Life Determination According To International Guidelines at Donna Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of The duration during which a drug maintains at least 90% of its labeled potency under specified conditions. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. However, product maturation may necessitate shelf life extension, requiring new stability studies. In vitro dissolution testing for solid dosage forms. Methods for the preparation of. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.
From www.linkedin.com
A Guide to Calculating the Shelf Life of Foods Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of Early detection and corrective action can lead to a drug. However, product maturation may necessitate shelf life extension, requiring new stability studies. Stability is determined by evaluation of the quality parameters with time under the influence of a variety of. In vitro dissolution testing for solid dosage forms. The duration during which a drug maintains at least 90% of its. Shelf Life Determination Of Pharmaceutical Dosage Form Uses The Principle Of.