Titration Endpoint Ph . Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Using a ph probe and using a colour indicator. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The reaction can be represented as:
from www.chegg.com
The reaction can be represented as: Using a ph probe and using a colour indicator. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base:
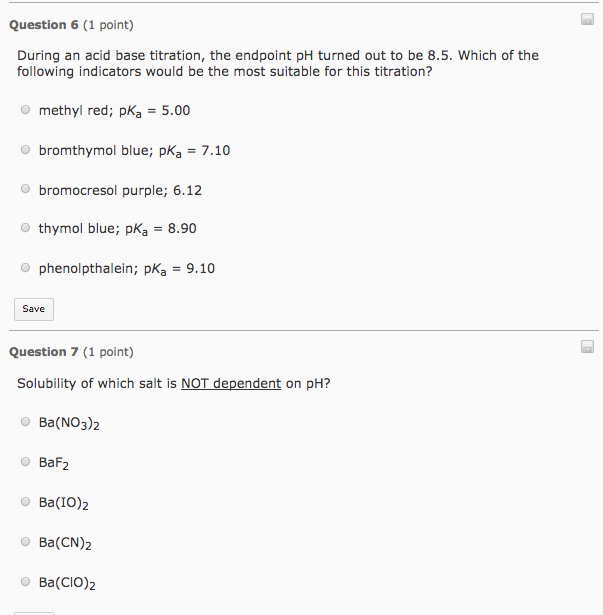
Solved During an acid base titration, the endpoint pH turned
Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The reaction can be represented as: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point.
From www.animalia-life.club
Endpoint Titration Titration Endpoint Ph calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: for a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator.. Titration Endpoint Ph.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube Titration Endpoint Ph 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The reaction can be represented as: for a ph titration (between an acid and a base), there are two common ways to find the endpoint: Consider the titration of 25.00. Titration Endpoint Ph.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Titration Endpoint Ph for a ph titration (between an acid and a base), there are two common ways to find the endpoint: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: in this section we will learn how to. Titration Endpoint Ph.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The reaction can be represented as: for a ph titration (between an acid and a base), there are two common ways to find the endpoint: calculate the ph for the strong acid/strong base titration between 50.0. Titration Endpoint Ph.
From ck12.org
Titration CK12 Foundation Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The reaction can be represented as: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Consider. Titration Endpoint Ph.
From slideplayer.com
Titration Acids & Bases. ppt download Titration Endpoint Ph The reaction can be represented as: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Using a ph probe and using a colour indicator. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. in the overview to. Titration Endpoint Ph.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Endpoint Ph Using a ph probe and using a colour indicator. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: The reaction can be represented as: Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h. Titration Endpoint Ph.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Endpoint Ph Using a ph probe and using a colour indicator. The reaction can be represented as: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: for a ph titration (between an acid and a base), there are two. Titration Endpoint Ph.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Endpoint Ph calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. in the overview to this chapter we noted that a. Titration Endpoint Ph.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Titration Endpoint Ph The reaction can be represented as: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. 0.00 ml, 15.0. Titration Endpoint Ph.
From animalia-life.club
Endpoint Titration Titration Endpoint Ph calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. for a ph titration (between an acid and a base),. Titration Endpoint Ph.
From www.chegg.com
Solved The titration shown in the plot has an equivalence Titration Endpoint Ph The reaction can be represented as: Using a ph probe and using a colour indicator. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. for a ph titration. Titration Endpoint Ph.
From www.animalia-life.club
Endpoint Titration Titration Endpoint Ph The reaction can be represented as: in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. for a ph titration (between an acid and a base), there are two common ways. Titration Endpoint Ph.
From saylordotorg.github.io
AcidBase Titrations Titration Endpoint Ph Using a ph probe and using a colour indicator. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: for a ph titration (between an acid and a base), there are two common ways to find the endpoint:. Titration Endpoint Ph.
From mungfali.com
Endpoint Titration Curve Titration Endpoint Ph in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The reaction can be represented as: Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. in this section we will learn. Titration Endpoint Ph.
From www.chegg.com
Solved During an acid base titration, the endpoint pH turned Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Using a ph probe and using a colour indicator. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: Consider the titration of 25.00 ml of 0.100 m. Titration Endpoint Ph.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Endpoint Ph 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Using a ph probe and using a colour indicator.. Titration Endpoint Ph.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 Titration Endpoint Ph in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. in this section we will learn how to calculate the. Titration Endpoint Ph.
From www.slideserve.com
PPT Buffers and Acid/Base Titration PowerPoint Presentation, free Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Using a ph probe and using a colour indicator. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. for a ph titration (between an acid and a base), there are two common ways to find the endpoint:. Titration Endpoint Ph.
From www.animalia-life.club
Endpoint Titration Titration Endpoint Ph in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Using a ph probe and using a colour indicator. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base:. Titration Endpoint Ph.
From chemistry.stackexchange.com
Shape of WeakStrong AcidBase Titration Chemistry Stack Exchange Titration Endpoint Ph The reaction can be represented as: in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: in this section we will learn how to calculate the ph of an. Titration Endpoint Ph.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Titration Endpoint Ph 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. in this section. Titration Endpoint Ph.
From www.slideserve.com
PPT Titration Curves PowerPoint Presentation, free download ID3970559 Titration Endpoint Ph Using a ph probe and using a colour indicator. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. calculate. Titration Endpoint Ph.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Endpoint Ph in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. Using a ph probe and using a colour indicator. The reaction can be. Titration Endpoint Ph.
From www.chegg.com
Titration A, endpoint pH = Titration B, endpoint pH Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh.. Titration Endpoint Ph.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3. Titration Endpoint Ph.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Endpoint Ph in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Using a ph probe and using a colour indicator. calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of. Titration Endpoint Ph.
From slideplayer.com
Ch. 15 & 16 Acids & Bases III. Titration (p ) ppt download Titration Endpoint Ph Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. for a ph titration (between an acid and a base), there are two common ways to find the endpoint:. Titration Endpoint Ph.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Titration Endpoint Ph Using a ph probe and using a colour indicator. The reaction can be represented as: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. 0.00 ml, 15.0 ml, 25.0. Titration Endpoint Ph.
From www.youtube.com
17.3 Weak Acid Strong Base Titration pH at Endpoint YouTube Titration Endpoint Ph calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The reaction can be represented as: in this. Titration Endpoint Ph.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Endpoint Ph for a ph titration (between an acid and a base), there are two common ways to find the endpoint: calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml.. Titration Endpoint Ph.
From schoolbag.info
Titration and Buffers Acids and Bases Titration Endpoint Ph 0.00 ml, 15.0 ml, 25.0 ml, and 40.0 ml. for a ph titration (between an acid and a base), there are two common ways to find the endpoint: Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. in this section we will learn how to calculate the ph of. Titration Endpoint Ph.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Titration Endpoint Ph for a ph titration (between an acid and a base), there are two common ways to find the endpoint: in the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and. Titration Endpoint Ph.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Titration Endpoint Ph calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. in the overview to this chapter. Titration Endpoint Ph.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Endpoint Ph Consider the titration of 25.00 ml of 0.100 m ch 3 co 2 h with 0.100 m naoh. in this section we will learn how to calculate the ph of an analyte solution throughout the titration, and use these. The reaction can be represented as: for a ph titration (between an acid and a base), there are two. Titration Endpoint Ph.