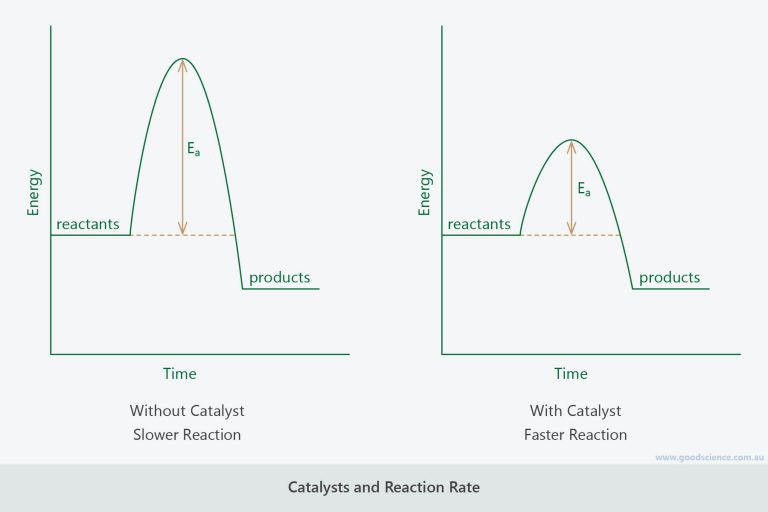
How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize . It assumes that you are already familiar with basic ideas. Of catalyst is needed to increase the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. →how does a catalyst affect the rate of a reaction? This means that fewer collisions. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. Only a very small mass of catalyst is needed to increase the rate of a reaction. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. Mass is measured in kilograms (kg) or grams (g). This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. However, not all reactions have suitable catalysts. However, not all reactions have suitable catalysts. However, not all reactions have suitable.
from www.goodscience.com.au
However, not all reactions have suitable. Mass is measured in kilograms (kg) or grams (g). Of catalyst is needed to increase the rate of a reaction. →how does a catalyst affect the rate of a reaction? This means that fewer collisions. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts.
Factors that Affect Rate of Reaction Good Science
How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Mass is measured in kilograms (kg) or grams (g). A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. Only a very small mass of catalyst is needed to increase the rate of a reaction. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. The effect of catalysts on reaction rates. This means that fewer collisions. However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. →how does a catalyst affect the rate of a reaction? It assumes that you are already familiar with basic ideas. Of catalyst is needed to increase the rate of a reaction. Mass is measured in kilograms (kg) or grams (g). However, not all reactions have suitable catalysts. However, not all reactions have suitable.
From byjus.com
How does a catalyst increase the rate of a reaction? How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize However, not all reactions have suitable catalysts. The effect of catalysts on reaction rates. Only a very small mass of catalyst is needed to increase the rate of a reaction. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. →how does a catalyst affect the rate of a reaction? This page describes. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.youtube.com
How Catalysts Affect Rate Of Reaction GCSE Chemistry How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize This means that fewer collisions. →how does a catalyst affect the rate of a reaction? Only a very small mass of catalyst is needed to increase the rate of a reaction. The effect of catalysts on reaction rates. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts work by attracting reactant molecules. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.youtube.com
GCSE Chemistry Factors Affecting the Rate of Reaction 47 YouTube How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize The effect of catalysts on reaction rates. Mass is measured in kilograms (kg) or grams (g). This means that fewer collisions. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. This gcse bbc. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.pinterest.com
the text bbc gcse bitesize biological catalysts on a blue How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. It assumes that you are already familiar with basic ideas. Of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.slideshare.net
Biology 2.4 How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. However, not all reactions have suitable catalysts. Only a very small mass of catalyst is needed to increase the rate of a reaction. Only. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction.. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From exojqwcbb.blob.core.windows.net
Catalyst Chemistry Symbol at Brenda Jones blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. →how does a catalyst affect the rate of a reaction? However, not all reactions. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize However, not all reactions have suitable catalysts. However, not all reactions have suitable. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Only a very small mass of. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Of catalyst is needed to increase the rate of a reaction. Mass is measured in kilograms (kg) or grams (g). However, not all reactions have suitable. This means that fewer collisions. This page describes and explains the way that adding a catalyst affects the. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.youtube.com
Factors Affecting the Rate of Reaction Catalyst Rate of Reaction How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize However, not all reactions have suitable catalysts. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. Of catalyst is needed to increase the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst affects the rate of. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize This page describes and explains the way that adding a catalyst affects the rate of a reaction. →how does a catalyst affect the rate of a reaction? Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.compoundchem.com
Making Reactions Faster Factors Affecting Rates of Reaction Compound How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize However, not all reactions have suitable catalysts. →how does a catalyst affect the rate of a reaction? This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. It assumes that you are already familiar with basic ideas. A catalyst affects the rate of a reaction by lowering the activation energy required for the. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.bbc.co.uk
BBC Two KS4 Curriculum Bites, Science 1416, Collision theory and how How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Mass is measured in kilograms (kg) or grams (g). This means that fewer collisions. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. It assumes that you are already familiar with basic ideas. A catalyst affects the rate of a reaction by lowering the activation energy required for the. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.numerade.com
SOLVEDDoes a catalyst increase reaction rate by the same means as a How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize →how does a catalyst affect the rate of a reaction? This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. This means that fewer collisions. However, not all reactions have suitable catalysts. However, not all reactions have suitable. However, not all reactions have suitable catalysts. Catalysts. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize The effect of catalysts on reaction rates. However, not all reactions have suitable. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. →how does a catalyst affect the rate of a reaction? This page describes and explains the way that adding a catalyst affects the rate of a reaction.. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize However, not all reactions have suitable catalysts. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. →how does a catalyst affect the rate of a reaction? However, not all reactions have suitable catalysts. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This means. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Mass is measured in kilograms (kg) or grams (g). Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. However, not all reactions have suitable. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. Only a. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.bbc.co.uk
BBC Two Bitesize Science, Rates of reactions How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize It assumes that you are already familiar with basic ideas. Only a very small mass of catalyst is needed to increase the rate of a reaction. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. However, not all reactions have suitable catalysts. This page describes and explains the way that adding a. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From ar.inspiredpencil.com
Catalyst Reaction How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize The effect of catalysts on reaction rates. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This means that fewer collisions. A catalyst affects the rate of a reaction by lowering the. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. It assumes that you are already familiar with basic ideas. Of catalyst is needed to increase the rate of a reaction.. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From socratic.org
What will occur if a catalyst is added to a reaction mixture? Socratic How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. The effect of catalysts on reaction rates. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. This means that fewer collisions. Only a very small mass of catalyst is needed to increase the rate of a. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. The effect of catalysts on reaction rates. →how does a catalyst affect the rate of a reaction? Mass is measured in kilograms (kg) or grams (g). This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. However,. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From joiievmpw.blob.core.windows.net
Low Enzyme Production And Metabolism at Rose Cooper blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize It assumes that you are already familiar with basic ideas. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. This page describes and explains the way that adding a catalyst affects the. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. Of catalyst is needed to increase the rate of a reaction. This means that fewer collisions. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. The effect of catalysts on. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From 2ly1udq7schematic.z4.web.core.windows.net
Exothermic Potential Energy Diagram Labeled How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize However, not all reactions have suitable catalysts. →how does a catalyst affect the rate of a reaction? This means that fewer collisions. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. This page describes and explains the way that adding a catalyst affects the rate of a reaction. However,. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.nagwa.com
Question Video Describing the Effect of Catalysts on Reaction Rates How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Of catalyst is needed to increase the rate of a reaction. This means that fewer collisions. This gcse bbc bitesize video is from the original programmes from 2000 that were broadcast on bbc2. Only a very small mass of catalyst is needed to increase the rate of a reaction. Catalysts work by attracting reactant molecules on to the surface and. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From joijjhybw.blob.core.windows.net
Catalyst Affect Rate Of Reaction at Carmelo Gentry blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Mass is measured in kilograms (kg) or grams (g). Of catalyst is needed to increase the rate of a reaction. The effect of catalysts on reaction rates. This means that fewer collisions. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable. A catalyst affects the rate of. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.chegg.com
Solved How are the following aspects of a reaction affected How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. However, not all reactions have suitable. However, not all reactions have suitable catalysts. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. →how does a catalyst affect the rate of. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From klamsflsd.blob.core.windows.net
Catalyst Change A Reaction at Rebecca Miller blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize It assumes that you are already familiar with basic ideas. Only a very small mass of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable catalysts. A catalyst affects the rate of a reaction by lowering the activation energy required for the reaction to occur. Catalysts work by attracting reactant molecules on to. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Mass is measured in kilograms (kg) or grams (g). The effect of catalysts on reaction rates. →how does a catalyst affect the rate of a reaction? This means. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From exoigvwpw.blob.core.windows.net
The Effect Of Catalysts On Rate Of Reaction at Clyde Berry blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Only a very small mass of catalyst is needed to increase the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. Mass is measured in kilograms (kg) or grams (g). This means that fewer collisions. →how does a catalyst affect the rate of a reaction? Catalysts work by attracting. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From loeljyzzv.blob.core.windows.net
Catalyst Chemical Reaction Rate at Leandro Jordan blog How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Of catalyst is needed to increase the rate of a reaction. However, not all reactions have suitable. However, not all reactions have suitable catalysts. →how does a catalyst affect the rate of a reaction? It assumes that you are already familiar with basic ideas. Only a very small mass of catalyst is needed to increase the rate of a reaction.. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.
From www.onlinebiologynotes.com
Rate of enzyme reactions and factor affecting the rate of enzyme How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize Mass is measured in kilograms (kg) or grams (g). However, not all reactions have suitable catalysts. Catalysts work by attracting reactant molecules on to the surface and so providing an alternate reaction pathway of lower energy. The effect of catalysts on reaction rates. However, not all reactions have suitable. Of catalyst is needed to increase the rate of a reaction.. How Does A Catalyst Increase The Rate Of A Reaction Bbc Bitesize.