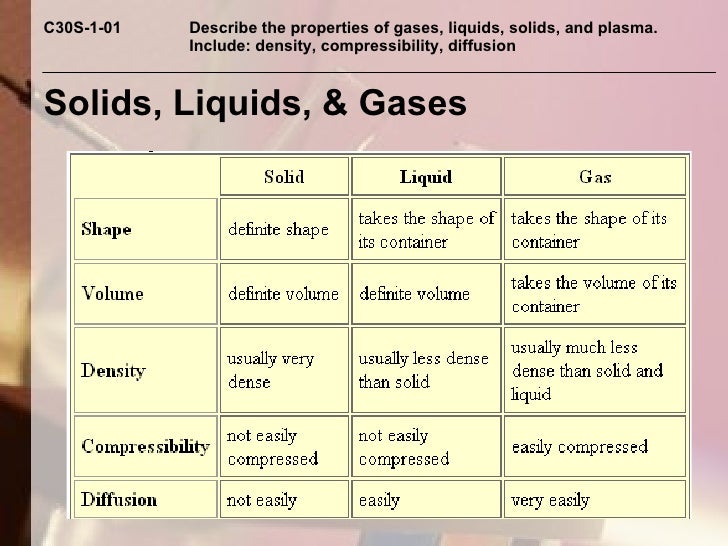
Solids And Liquids Hard To Compress . High pressure will lead to a decrease in volume, even if. The atoms, ions, or molecules that make up the solid or liquid are very close together. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). There is no space between the individual particles, so they cannot. Solids and liquids however are not as compressible. However, they are not entirely incompressible! The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. However, it requires a great deal of pressure to accomplish a little. In gases, they aren't and. The answer is yes, you can compress water, or almost any material.
from www.slideshare.net
There is no space between the individual particles, so they cannot. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). The answer is yes, you can compress water, or almost any material. However, it requires a great deal of pressure to accomplish a little. However, they are not entirely incompressible! High pressure will lead to a decrease in volume, even if. In gases, they aren't and. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. The atoms, ions, or molecules that make up the solid or liquid are very close together. Solids and liquids however are not as compressible.
Unit 1 Notes
Solids And Liquids Hard To Compress Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, they are not entirely incompressible! However, it requires a great deal of pressure to accomplish a little. The answer is yes, you can compress water, or almost any material. There is no space between the individual particles, so they cannot. The atoms, ions, or molecules that make up the solid or liquid are very close together. In gases, they aren't and. High pressure will lead to a decrease in volume, even if. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. Solids and liquids however are not as compressible.
From loezttyas.blob.core.windows.net
What Gas Can Be Compressed at Norman Williams blog Solids And Liquids Hard To Compress However, they are not entirely incompressible! High pressure will lead to a decrease in volume, even if. Solids and liquids however are not as compressible. In gases, they aren't and. The atoms, ions, or molecules that make up the solid or liquid are very close together. There is no space between the individual particles, so they cannot. However, it requires. Solids And Liquids Hard To Compress.
From mavink.com
Solids Liquids And Gases Gif Solids And Liquids Hard To Compress The answer is yes, you can compress water, or almost any material. However, they are not entirely incompressible! The atoms, ions, or molecules that make up the solid or liquid are very close together. There is no space between the individual particles, so they cannot. High pressure will lead to a decrease in volume, even if. However, it requires a. Solids And Liquids Hard To Compress.
From dxojeueds.blob.core.windows.net
Solids Liquids And Gases Third Grade at Ann Trotter blog Solids And Liquids Hard To Compress However, they are not entirely incompressible! The atoms, ions, or molecules that make up the solid or liquid are very close together. There is no space between the individual particles, so they cannot. The answer is yes, you can compress water, or almost any material. High pressure will lead to a decrease in volume, even if. In gases, they aren't. Solids And Liquids Hard To Compress.
From www.exploringnature.org
Phases of Matter Gas, Liquids, Solids Solids And Liquids Hard To Compress The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. In gases, they aren't and. The answer is yes, you can compress water, or almost any material. However, they are not entirely incompressible! Solids and liquids however are not as compressible. Something is usually described as a solid. Solids And Liquids Hard To Compress.
From slideplayer.com
Solids Liquids Gases TB p ppt download Solids And Liquids Hard To Compress High pressure will lead to a decrease in volume, even if. In gases, they aren't and. The atoms, ions, or molecules that make up the solid or liquid are very close together. The answer is yes, you can compress water, or almost any material. There is no space between the individual particles, so they cannot. Something is usually described as. Solids And Liquids Hard To Compress.
From joisrkcqc.blob.core.windows.net
Which Properties Do All Solids Liquids And Gases Have at Estelle Lewis blog Solids And Liquids Hard To Compress High pressure will lead to a decrease in volume, even if. The atoms, ions, or molecules that make up the solid or liquid are very close together. However, they are not entirely incompressible! In gases, they aren't and. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion.. Solids And Liquids Hard To Compress.
From www.youtube.com
Fluid Mechanics Introduction Compressibility of Fluids YouTube Solids And Liquids Hard To Compress The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. High pressure will lead to a decrease in volume, even if. However, they are not entirely incompressible! The atoms, ions, or molecules that make up the solid or liquid are very close together. Something is usually described as. Solids And Liquids Hard To Compress.
From www.youtube.com
Gas can compress YouTube Solids And Liquids Hard To Compress Solids and liquids however are not as compressible. In gases, they aren't and. The answer is yes, you can compress water, or almost any material. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). There is no space between the individual particles, so they cannot. The atoms, ions, or. Solids And Liquids Hard To Compress.
From www.slideshare.net
Unit 1 Notes Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a little. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, they are not entirely incompressible! The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion.. Solids And Liquids Hard To Compress.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a little. There is no space between the individual particles, so they cannot. In gases, they aren't and. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). Solids and liquids however are not as compressible. High pressure will lead. Solids And Liquids Hard To Compress.
From www.chegg.com
Solved Which of the following statements is/are true about Solids And Liquids Hard To Compress The atoms, ions, or molecules that make up the solid or liquid are very close together. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). The answer. Solids And Liquids Hard To Compress.
From goformative.com
Solids, Liquids, Gases Formative Trinity Library Formative Solids And Liquids Hard To Compress Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). There is no space between the individual particles, so they cannot. The answer is yes, you can compress water, or almost any material. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap. Solids And Liquids Hard To Compress.
From dxoeeuvov.blob.core.windows.net
Solid Liquid Gas Examples Atoms at Lucretia Carroll blog Solids And Liquids Hard To Compress The answer is yes, you can compress water, or almost any material. High pressure will lead to a decrease in volume, even if. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, it requires a great deal of pressure to accomplish a little. However, they are not entirely. Solids And Liquids Hard To Compress.
From www.slideserve.com
PPT Solids, Liquids, and Gases PowerPoint Presentation, free download Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a little. The answer is yes, you can compress water, or almost any material. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, they are not entirely incompressible! High pressure will lead to a decrease in volume, even. Solids And Liquids Hard To Compress.
From www.scribd.com
PDF Study of Diffusion of Solids in Liquids Compress PDF Solubility Solids And Liquids Hard To Compress Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, it requires a great deal of pressure to accomplish a little. The answer is yes, you can compress water, or almost any material. The atoms, ions, or molecules that make up the solid or liquid are very close together.. Solids And Liquids Hard To Compress.
From www.toppr.com
Difference Between Solid Liquid and Gas with Definitions Solids And Liquids Hard To Compress The atoms, ions, or molecules that make up the solid or liquid are very close together. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). In gases, they aren't and. Solids and liquids however are not as compressible. However, it requires a great deal of pressure to accomplish a. Solids And Liquids Hard To Compress.
From simply.science
Liquids Solids And Liquids Hard To Compress The answer is yes, you can compress water, or almost any material. However, it requires a great deal of pressure to accomplish a little. Solids and liquids however are not as compressible. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. High pressure will lead to a. Solids And Liquids Hard To Compress.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solids And Liquids Hard To Compress Solids and liquids however are not as compressible. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. The answer is yes, you can compress water, or almost any material. In gases, they aren't and. Something is usually described as a solid if it can hold its own. Solids And Liquids Hard To Compress.
From www.pinterest.ph
Matter Difference between solid liquid and gas Why solids liquids and Solids And Liquids Hard To Compress The answer is yes, you can compress water, or almost any material. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). However, they are not entirely incompressible! However, it requires a great deal of pressure to accomplish a little. In gases, they aren't and. The solid is not compressible. Solids And Liquids Hard To Compress.
From loebhlsuw.blob.core.windows.net
Are Minerals Solid Liquid And Gas at Raymond Parkman blog Solids And Liquids Hard To Compress In gases, they aren't and. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). There is no space between the individual particles, so they cannot. The answer is yes, you can compress water, or almost any material. However, it requires a great deal of pressure to accomplish a little.. Solids And Liquids Hard To Compress.
From www.tutorix.com
How do solids liquids and gases differ in shape an Tutorix Solids And Liquids Hard To Compress There is no space between the individual particles, so they cannot. High pressure will lead to a decrease in volume, even if. However, it requires a great deal of pressure to accomplish a little. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). The solid is not compressible because. Solids And Liquids Hard To Compress.
From www.slideserve.com
PPT Unit 3 Fluids & Dynamics PowerPoint Presentation, free download Solids And Liquids Hard To Compress Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). The answer is yes, you can compress water, or almost any material. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. In gases, they aren't and. Solids. Solids And Liquids Hard To Compress.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Solids And Liquids Hard To Compress The atoms, ions, or molecules that make up the solid or liquid are very close together. Solids and liquids however are not as compressible. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. However, they are not entirely incompressible! In gases, they aren't and. The answer is. Solids And Liquids Hard To Compress.
From www.crbgroup.com
Oral solid dosage manufacturing CRB Solids And Liquids Hard To Compress The answer is yes, you can compress water, or almost any material. There is no space between the individual particles, so they cannot. In gases, they aren't and. However, they are not entirely incompressible! Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). Solids and liquids however are not. Solids And Liquids Hard To Compress.
From www.youtube.com
Properties of liquids How to show that liquids do not have fixed Solids And Liquids Hard To Compress However, they are not entirely incompressible! Solids and liquids however are not as compressible. The atoms, ions, or molecules that make up the solid or liquid are very close together. There is no space between the individual particles, so they cannot. High pressure will lead to a decrease in volume, even if. In gases, they aren't and. However, it requires. Solids And Liquids Hard To Compress.
From byjus.com
Activity To show that solids and liquids cannot be compressed but gases Solids And Liquids Hard To Compress There is no space between the individual particles, so they cannot. High pressure will lead to a decrease in volume, even if. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and. Solids And Liquids Hard To Compress.
From www.ogj.com
Range Resources inches up 2024 total production guidance Oil & Gas Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a little. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. However, they are not entirely incompressible! High pressure will lead to a decrease in volume, even if. There is no space between the individual particles, so. Solids And Liquids Hard To Compress.
From www.teachoo.com
Activity to Show Gases can be Compressed but Solids and Liquids cannot Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a little. There is no space between the individual particles, so they cannot. The atoms, ions, or molecules that make up the solid or liquid are very close together. Solids and liquids however are not as compressible. Something is usually described as a solid if it can hold its own. Solids And Liquids Hard To Compress.
From primaryleap.co.uk
Chemistry Liquids Level 1 activity for kids PrimaryLeap.co.uk Solids And Liquids Hard To Compress Solids and liquids however are not as compressible. High pressure will lead to a decrease in volume, even if. However, they are not entirely incompressible! The answer is yes, you can compress water, or almost any material. In gases, they aren't and. There is no space between the individual particles, so they cannot. The solid is not compressible because as. Solids And Liquids Hard To Compress.
From www.youtube.com
Activity to show that gases can be compressed more easily than liquids Solids And Liquids Hard To Compress However, it requires a great deal of pressure to accomplish a little. Something is usually described as a solid if it can hold its own shape and is hard to compress (squash). Solids and liquids however are not as compressible. In gases, they aren't and. High pressure will lead to a decrease in volume, even if. The atoms, ions, or. Solids And Liquids Hard To Compress.
From www.bariatricfusion.com
2 Week Pre Op Gastric Sleeve Diet Essential Guide Solids And Liquids Hard To Compress In gases, they aren't and. However, they are not entirely incompressible! The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. The atoms, ions, or molecules that make up the solid or liquid are very close together. Solids and liquids however are not as compressible. High pressure will. Solids And Liquids Hard To Compress.
From www.slideserve.com
PPT Particle model of solids, liquids and gases PowerPoint Solids And Liquids Hard To Compress The answer is yes, you can compress water, or almost any material. The atoms, ions, or molecules that make up the solid or liquid are very close together. Solids and liquids however are not as compressible. High pressure will lead to a decrease in volume, even if. However, it requires a great deal of pressure to accomplish a little. The. Solids And Liquids Hard To Compress.
From www.askiitians.com
Classification of Solids Study Material for IIT JEE askIITians Solids And Liquids Hard To Compress The atoms, ions, or molecules that make up the solid or liquid are very close together. In gases, they aren't and. The solid is not compressible because as the pressure is increased, the atomic orbitals tend to overlap and due to pauli exclusion. However, it requires a great deal of pressure to accomplish a little. Solids and liquids however are. Solids And Liquids Hard To Compress.
From www.pinterest.com
Liquid Definition Examples of Liquids Solid liquid gas, States of Solids And Liquids Hard To Compress The atoms, ions, or molecules that make up the solid or liquid are very close together. In gases, they aren't and. However, it requires a great deal of pressure to accomplish a little. Solids and liquids however are not as compressible. The answer is yes, you can compress water, or almost any material. The solid is not compressible because as. Solids And Liquids Hard To Compress.
From thepiquelab.com
Properties of Matter What is Compression? Primary School Science Solids And Liquids Hard To Compress However, they are not entirely incompressible! Solids and liquids however are not as compressible. The answer is yes, you can compress water, or almost any material. However, it requires a great deal of pressure to accomplish a little. The atoms, ions, or molecules that make up the solid or liquid are very close together. Something is usually described as a. Solids And Liquids Hard To Compress.