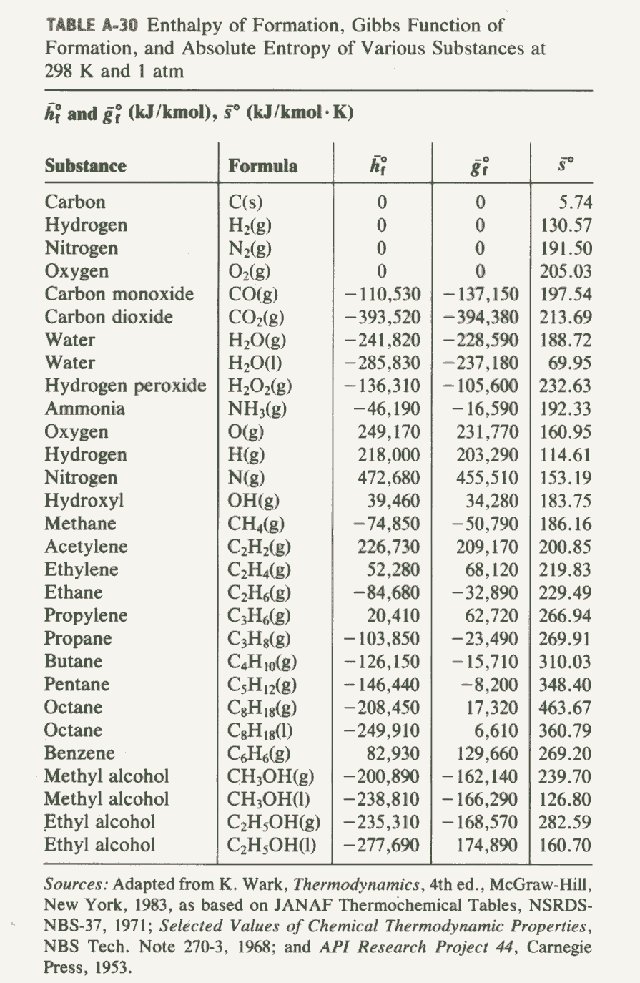
Standard Heat Formation Table . The elemental form of each atom is that with the lowest enthalpy in the standard state. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. \[\delta h_{reaction}^o = \sum {\delta. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. The standard state heat of formation for the elemental form of each atom is zero. To find the δh reaction o, use the formula for the standard enthalpy change of formation:
from rayb78.github.io
The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for the elemental form of each atom is zero. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To find the δh reaction o, use the formula for the standard enthalpy change of formation: This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+.
Heat Of Formation Chart
Standard Heat Formation Table The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. \[\delta h_{reaction}^o = \sum {\delta. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The elemental form of each atom is that with the lowest enthalpy in the standard state. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. The standard state heat of formation for the elemental form of each atom is zero.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Heat Formation Table The standard state heat of formation for the elemental form of each atom is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The elemental form of each atom is that with the lowest enthalpy in the standard state. Also called standard enthalpy of formation,. Standard Heat Formation Table.
From quizzdbcrnosivimrwg.z13.web.core.windows.net
Heat Of Formation Worksheets Standard Heat Formation Table Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from. Standard Heat Formation Table.
From learningschoolgraciauwb.z4.web.core.windows.net
Heat Of Reaction Formula Standard Heat Formation Table 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard state heat of formation for the elemental form of each atom is zero.. Standard Heat Formation Table.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Formation Table The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy.. Standard Heat Formation Table.
From cepbtpfh.blob.core.windows.net
Standard Heat Of Formation Hydrogen Peroxide at John Ahmed blog Standard Heat Formation Table To find the δh reaction o, use the formula for the standard enthalpy change of formation: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and. Standard Heat Formation Table.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Heat Formation Table To find the δh reaction o, use the formula for the standard enthalpy change of formation: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The. Standard Heat Formation Table.
From www.chegg.com
Learning GoalTo understand how standard enthalpy of Standard Heat Formation Table 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. Standard Heat Formation Table.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Heat Formation Table \[\delta h_{reaction}^o = \sum {\delta. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard state heat of formation for the elemental form of each atom is zero. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when. Standard Heat Formation Table.
From zeviernswenson.blogspot.com
Standard Enthalpy of Formation ZeviernSwenson Standard Heat Formation Table The elemental form of each atom is that with the lowest enthalpy in the standard state. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. The. Standard Heat Formation Table.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Formation Table The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. This table lists the standard enthalpies (δh°), the free. Standard Heat Formation Table.
From www.semanticscholar.org
[PDF] Heats of combustion of high temperature polymers Semantic Scholar Standard Heat Formation Table The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation. Standard Heat Formation Table.
From www.researchgate.net
Heat of formation and enthalpy data for slag compounds Enthalpy of Standard Heat Formation Table To find the δh reaction o, use the formula for the standard enthalpy change of formation: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. 193. Standard Heat Formation Table.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Formation Table 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. \[\delta h_{reaction}^o = \sum {\delta. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. The standard state heat of formation for the elemental form of. Standard Heat Formation Table.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Formation Table \[\delta h_{reaction}^o = \sum {\delta. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in. Standard Heat Formation Table.
From mungfali.com
Enthalpies Of Formation Table Standard Heat Formation Table 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard state heat of formation for the elemental form of each atom is zero. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,.. Standard Heat Formation Table.
From www.semanticscholar.org
[PDF] Largescale calculations of gas phase thermochemistry Enthalpy Standard Heat Formation Table The elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of. Standard Heat Formation Table.
From lessonlibnurselings.z21.web.core.windows.net
Heats Of Formation Equation Standard Heat Formation Table \[\delta h_{reaction}^o = \sum {\delta. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k). Standard Heat Formation Table.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Heat Formation Table This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The elemental form of each atom is that with the lowest enthalpy in the standard state. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation,. Standard Heat Formation Table.
From worksheetmediasnouts.z13.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Formation Table To find the δh reaction o, use the formula for the standard enthalpy change of formation: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the. Standard Heat Formation Table.
From www.semanticscholar.org
Table 3 from Standard thermodynamic properties of H3PO4(aq) over a wide Standard Heat Formation Table The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Also called standard enthalpy of formation, the molar. Standard Heat Formation Table.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Formation Table 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. \[\delta h_{reaction}^o = \sum {\delta. Also called standard enthalpy of formation, the molar heat of. Standard Heat Formation Table.
From mungfali.com
Enthalpies Of Formation Chart Standard Heat Formation Table \[\delta h_{reaction}^o = \sum {\delta. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The elemental form of each atom is that with the lowest enthalpy in the standard state.. Standard Heat Formation Table.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Heat Formation Table This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The elemental form of each atom is that with the lowest enthalpy in the standard state. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and. Standard Heat Formation Table.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Standard Heat Formation Table The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. The elemental form of each atom is that with the lowest enthalpy in the standard state. \[\delta h_{reaction}^o = \sum {\delta. This table lists the standard enthalpies (δh°), the free energies. Standard Heat Formation Table.
From wisataparatravell.blogspot.com
Standard Heats Of Formation Standard Enthalpies Of Formation And The Standard Heat Formation Table \[\delta h_{reaction}^o = \sum {\delta. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. To find the δh reaction o, use the formula for the standard enthalpy change of formation: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Heat Formation Table.
From boomeria.org
Ch 20 Standard Heat Formation Table Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. \[\delta h_{reaction}^o = \sum {\delta. To find the δh reaction o, use the formula for the standard enthalpy change of formation: This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements. Standard Heat Formation Table.
From exoziqnph.blob.core.windows.net
Standard Heat Of Formation Co2 at Jonathan Blackburn blog Standard Heat Formation Table 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at. \[\delta h_{reaction}^o = \sum {\delta. The standard state. Standard Heat Formation Table.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Heat Formation Table 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To find the δh reaction o, use the formula for the standard enthalpy change of. Standard Heat Formation Table.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Heat Formation Table The standard state heat of formation for the elemental form of each atom is zero. To find the δh reaction o, use the formula for the standard enthalpy change of formation: \[\delta h_{reaction}^o = \sum {\delta. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Also. Standard Heat Formation Table.
From www.researchgate.net
Enthalpy of formation and heats of formation of main combustion Standard Heat Formation Table The elemental form of each atom is that with the lowest enthalpy in the standard state. The standard state heat of formation for the elemental form of each atom is zero. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. 193 rows in chemistry and thermodynamics,. Standard Heat Formation Table.
From rayb78.github.io
Heat Of Formation Chart Standard Heat Formation Table 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. This table lists the standard enthalpies (δh°), the free energies (δg°) of formation of compounds from elements in their standard states, and the. The standard state heat of formation for the elemental form of each atom is zero.. Standard Heat Formation Table.
From www.studocu.com
Heat of formation table STANDARD HEATS OF FORMATION FOR SELECTED Standard Heat Formation Table The elemental form of each atom is that with the lowest enthalpy in the standard state. To find the δh reaction o, use the formula for the standard enthalpy change of formation: Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. This table lists the standard enthalpies (δh°),. Standard Heat Formation Table.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Heat Formation Table The standard state heat of formation for the elemental form of each atom is zero. To find the δh reaction o, use the formula for the standard enthalpy change of formation: \[\delta h_{reaction}^o = \sum {\delta. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. The elemental form. Standard Heat Formation Table.
From www.slideshare.net
Heat of formation by reactions Standard Heat Formation Table The standard state heat of formation for the elemental form of each atom is zero. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. To find the δh reaction o, use the formula for the standard enthalpy change of formation: The standard enthalpy of formation, also known. Standard Heat Formation Table.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Heat Formation Table Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Heat Formation Table.