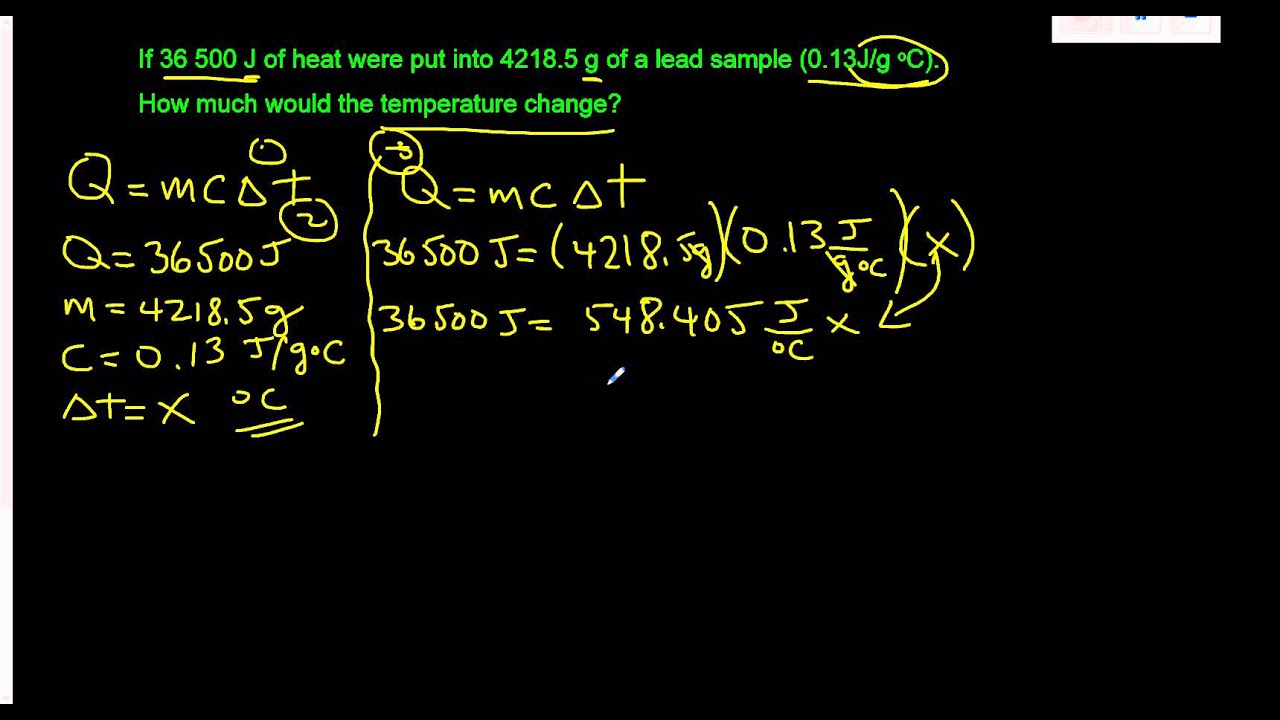What Does M Stand For In Q Mc T . Access the best chemistry resource at. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. When heat energy is added to a substance, the temperature will change by a certain amount. the quantitative relationship between heat transfer and temperature change contains all three factors: the heat capacity of an object made of a pure substance is, c=mc. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then. If the material an object is made of is uniform in. 🎯 want to ace chemistry? the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. Q = mcδt, where q is the.
from www.youtube.com
Access the best chemistry resource at. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. Q = mcδt, where q is the. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. the quantitative relationship between heat transfer and temperature change contains all three factors: When heat energy is added to a substance, the temperature will change by a certain amount. the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. the heat capacity of an object made of a pure substance is, c=mc. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then. 🎯 want to ace chemistry?
Solving for the variable ∆t in the Heat Formula Q = mc∆t YouTube
What Does M Stand For In Q Mc T 🎯 want to ace chemistry? the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. If the material an object is made of is uniform in. the heat capacity of an object made of a pure substance is, c=mc. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. Q = mcδt, where q is the. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. Access the best chemistry resource at. When heat energy is added to a substance, the temperature will change by a certain amount. the quantitative relationship between heat transfer and temperature change contains all three factors: Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then. 🎯 want to ace chemistry?
From www.youtube.com
2.0 Level q=mc∆T Practice YouTube What Does M Stand For In Q Mc T the heat capacity of an object made of a pure substance is, c=mc. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. the amount of heat gained or lost by a sample (q) can. What Does M Stand For In Q Mc T.
From indianexpress.com
MC Stan is the winner of Bigg Boss 16 The misfit who had the world What Does M Stand For In Q Mc T Q = mcδt, where q is the. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. Access the best chemistry resource at. When heat energy is added to a substance, the temperature will change by a. What Does M Stand For In Q Mc T.
From www.goeasytips.com
What Does M Mean In Math Go Easy Tips What Does M Stand For In Q Mc T the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then. If the material an object is made of is uniform. What Does M Stand For In Q Mc T.
From www.youtube.com
HTPIB14B Specific Heat and Q = mcT YouTube What Does M Stand For In Q Mc T When heat energy is added to a substance, the temperature will change by a certain amount. the heat capacity of an object made of a pure substance is, c=mc. the quantitative relationship between heat transfer and temperature change contains all three factors: Access the best chemistry resource at. the specific heat capacity, or simply specific heat (c). What Does M Stand For In Q Mc T.
From www.youtube.com
How to Use M+ M and MRC Buttons on Calculator YouTube What Does M Stand For In Q Mc T Access the best chemistry resource at. If the material an object is made of is uniform in. the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. the transfer of heat q that leads to a change δt in the temperature. What Does M Stand For In Q Mc T.
From f0rmule.blogspot.com
Formule Q = M.c.delta T Formule What Does M Stand For In Q Mc T If the material an object is made of is uniform in. the heat capacity of an object made of a pure substance is, c=mc. When heat energy is added to a substance, the temperature will change by a certain amount. the transfer of heat q that leads to a change δt in the temperature of a body with. What Does M Stand For In Q Mc T.
From www.youtube.com
Heat Flow, Heat Capacity, q = mC(delta)T YouTube What Does M Stand For In Q Mc T the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. 🎯 want to ace chemistry? the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to. What Does M Stand For In Q Mc T.
From www.newsdigging.com
Traffic cops to use cunning new way to catch unsuspecting motorists What Does M Stand For In Q Mc T If the material an object is made of is uniform in. When heat energy is added to a substance, the temperature will change by a certain amount. Q = mcδt, where q is the. the heat capacity of an object made of a pure substance is, c=mc. 🎯 want to ace chemistry? Access the best chemistry resource at.. What Does M Stand For In Q Mc T.
From www.youtube.com
3.0 Level q=mc∆T Practice YouTube What Does M Stand For In Q Mc T Q = mcδt, where q is the. When heat energy is added to a substance, the temperature will change by a certain amount. Access the best chemistry resource at. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the. What Does M Stand For In Q Mc T.
From haipernews.com
How To Calculate Q In Joules Haiper What Does M Stand For In Q Mc T the quantitative relationship between heat transfer and temperature change contains all three factors: 🎯 want to ace chemistry? the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. Access the best chemistry resource at. When heat energy is added to a substance, the temperature will change. What Does M Stand For In Q Mc T.
From ajpoisson.blogspot.com
chemistry calculating heat What Does M Stand For In Q Mc T the heat capacity of an object made of a pure substance is, c=mc. Q = mcδt, where q is the. the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. the transfer of heat q that leads to a change. What Does M Stand For In Q Mc T.
From dxojukgyb.blob.core.windows.net
What Does M Stand For In Latin at Sherry Savell blog What Does M Stand For In Q Mc T the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. the quantitative relationship between heat transfer and temperature change contains all three factors: Access the best chemistry resource at. 🎯 want to ace chemistry? When heat energy is added to a substance, the temperature will change. What Does M Stand For In Q Mc T.
From www.reddit.com
Avatar the Last Airbender Alphabet M stands for…? r What Does M Stand For In Q Mc T Access the best chemistry resource at. the heat capacity of an object made of a pure substance is, c=mc. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. Apply q reaction = (m) (c) (∆t),. What Does M Stand For In Q Mc T.
From www.showme.com
Using q=mc(TfTi) Dinosaur Education ShowMe What Does M Stand For In Q Mc T the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. When heat energy is added. What Does M Stand For In Q Mc T.
From www.youtube.com
What does the M stand for in AMP? YouTube What Does M Stand For In Q Mc T the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. 🎯 want to ace chemistry? If the material an object is made of is uniform in. the heat capacity of an object made of a pure substance is, c=mc. Apply q reaction = (m) (c) (∆t),. What Does M Stand For In Q Mc T.
From www.youtube.com
Solving for the variable ∆t in the Heat Formula Q = mc∆t YouTube What Does M Stand For In Q Mc T Access the best chemistry resource at. If the material an object is made of is uniform in. the quantitative relationship between heat transfer and temperature change contains all three factors: the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. Apply q reaction = (m) (c) (∆t),. What Does M Stand For In Q Mc T.
From www.showme.com
Solving q=mcat Science, Chemistry ShowMe What Does M Stand For In Q Mc T the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. 🎯 want to ace chemistry? the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to. What Does M Stand For In Q Mc T.
From www.youtube.com
Calculating thermal energy changes Q=mcdT YouTube What Does M Stand For In Q Mc T When heat energy is added to a substance, the temperature will change by a certain amount. 🎯 want to ace chemistry? the heat capacity of an object made of a pure substance is, c=mc. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q =. What Does M Stand For In Q Mc T.
From www.youtube.com
q=mct homework 2 YouTube What Does M Stand For In Q Mc T the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. the quantitative relationship between heat transfer and temperature change contains all three factors: 🎯 want to ace chemistry? Apply q reaction = (m) (c) (∆t), where delta t is an. What Does M Stand For In Q Mc T.
From allbizplan.ru
Math symbols What Does M Stand For In Q Mc T If the material an object is made of is uniform in. Q = mcδt, where q is the. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. the heat capacity of an object made of a pure substance is, c=mc. the transfer of heat q. What Does M Stand For In Q Mc T.
From www.gbu-presnenskij.ru
Heat Capacity And Calorimetry (practice) Khan Academy, 59 OFF What Does M Stand For In Q Mc T the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. the quantitative relationship between heat transfer and temperature change contains all three factors: Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is. What Does M Stand For In Q Mc T.
From exoyqkbqh.blob.core.windows.net
What Does M C Y T Mean at Scott b blog What Does M Stand For In Q Mc T the quantitative relationship between heat transfer and temperature change contains all three factors: Access the best chemistry resource at. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. the amount of heat gained or lost by a sample (q) can be calculated using the equation. What Does M Stand For In Q Mc T.
From fluentslang.com
What Does M Mean? Meaning, Uses and More FluentSlang What Does M Stand For In Q Mc T When heat energy is added to a substance, the temperature will change by a certain amount. Q = mcδt, where q is the. If the material an object is made of is uniform in. 🎯 want to ace chemistry? the quantitative relationship between heat transfer and temperature change contains all three factors: the heat capacity of an. What Does M Stand For In Q Mc T.
From f0rmule.blogspot.com
Formule Q = M.c.delta T Formule What Does M Stand For In Q Mc T the quantitative relationship between heat transfer and temperature change contains all three factors: 🎯 want to ace chemistry? the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. When heat energy is added to a substance, the temperature will change by a certain amount. Q =. What Does M Stand For In Q Mc T.
From www.showme.com
Chapter 10 Energy Problems (ME fusion/ vaporization) and Q=mc delta t What Does M Stand For In Q Mc T the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. If the material an object is made of is uniform in. 🎯 want to ace chemistry? the specific heat capacity, or simply specific heat (c). What Does M Stand For In Q Mc T.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube What Does M Stand For In Q Mc T Q = mcδt, where q is the. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the. What Does M Stand For In Q Mc T.
From www.youtube.com
11.2 q=mCT YouTube What Does M Stand For In Q Mc T Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then. Access the best chemistry resource at. If the material an object is made of is uniform in. the quantitative relationship between heat transfer and temperature change contains all three factors: When heat energy is added to a. What Does M Stand For In Q Mc T.
From studylibstearine.z21.web.core.windows.net
How To Find Qc What Does M Stand For In Q Mc T the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. When heat energy is added to a substance, the temperature will change by a certain amount. the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where. What Does M Stand For In Q Mc T.
From igvofficial.com
What Does M&M Stand For? What Does M Stand For In Q Mc T Access the best chemistry resource at. When heat energy is added to a substance, the temperature will change by a certain amount. the specific heat capacity, or simply specific heat (c) of a substance is the amount of heat energy required to raise. the transfer of heat q that leads to a change δt in the temperature of. What Does M Stand For In Q Mc T.
From amin2.blob.core.windows.net
Understanding The Acronym What Does "SFS" Stand For? What Does M Stand For In Q Mc T If the material an object is made of is uniform in. 🎯 want to ace chemistry? the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. When heat energy is added to a substance, the temperature will change by a certain. What Does M Stand For In Q Mc T.
From www.youtube.com
calculations of enthalpy; q= m c delta T YouTube What Does M Stand For In Q Mc T Access the best chemistry resource at. the transfer of heat q that leads to a change δt in the temperature of a body with mass m is q = m c δt, where c is the specific heat. If the material an object is made of is uniform in. the amount of heat gained or lost by a. What Does M Stand For In Q Mc T.
From www.youtube.com
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial What Does M Stand For In Q Mc T Q = mcδt, where q is the. the quantitative relationship between heat transfer and temperature change contains all three factors: If the material an object is made of is uniform in. the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the.. What Does M Stand For In Q Mc T.
From www.youtube.com
Using the formula q=mcΔT (Three examples) YouTube What Does M Stand For In Q Mc T the heat capacity of an object made of a pure substance is, c=mc. Access the best chemistry resource at. the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. If the material an object is made of is uniform in. . What Does M Stand For In Q Mc T.
From www.youtube.com
Heat Formula , Q=mc∆t YouTube What Does M Stand For In Q Mc T the amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcδt, where m is the mass of the. the quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the. the heat capacity of an object made of a pure. What Does M Stand For In Q Mc T.
From dxoasnubk.blob.core.windows.net
What Does Q T Stand For at Margie Fuller blog What Does M Stand For In Q Mc T Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then. Q = mcδt, where q is the. the quantitative relationship between heat transfer and temperature change contains all three factors: Access the best chemistry resource at. If the material an object is made of is uniform in.. What Does M Stand For In Q Mc T.