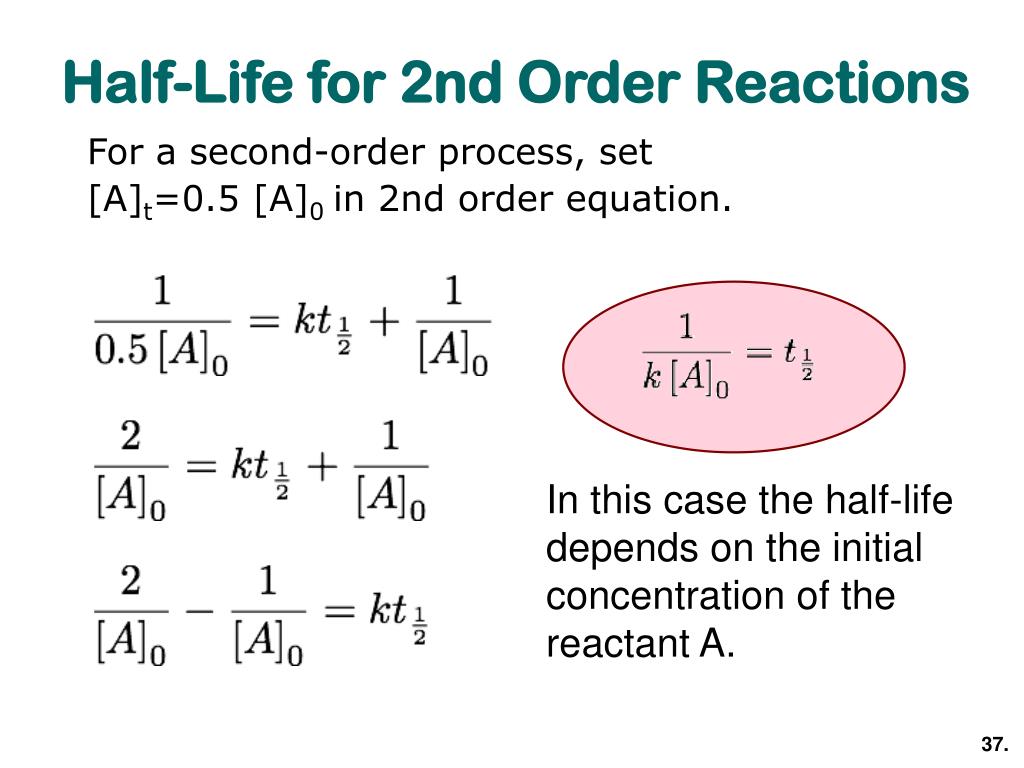
Half Life Equation Second Order . T ½ = 0.693 / k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: Consider the reaction \(2a \rightarrow p\): Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = [a o] / 2k. However, second order reactions decrease at a much faster rate as the graph shows. For a zero order reaction a products , rate = k:
from www.slideserve.com
T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: Consider the reaction \(2a \rightarrow p\): T ½ = 0.693 / k. However, second order reactions decrease at a much faster rate as the graph shows. For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time.
PPT Chemical Chapter 14 PowerPoint Presentation, free
Half Life Equation Second Order However, second order reactions decrease at a much faster rate as the graph shows. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = [a o] / 2k. For a second order reaction 2a products or a. However, second order reactions decrease at a much faster rate as the graph shows. Consider the reaction \(2a \rightarrow p\): For a zero order reaction a products , rate = k: T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]:
From www.netexplanations.com
Half life Formula Half Life Equation Second Order However, second order reactions decrease at a much faster rate as the graph shows. T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Time (\(t\)), which is similar to first order plots in that their slopes decrease. Half Life Equation Second Order.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free Half Life Equation Second Order $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a zero order reaction a products , rate = k: Consider the reaction \(2a \rightarrow p\): T ½ = 0.693 / k. However, second order reactions decrease at a much faster rate as the. Half Life Equation Second Order.
From www.vrogue.co
How To Calculate Half Life For Second Order Reaction vrogue.co Half Life Equation Second Order $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Consider the reaction \(2a \rightarrow p\): For a first order reaction a products , rate = k [a]: However, second order reactions decrease at a much faster rate as the graph shows. For a second order reaction 2a products or a. Time (\(t\)), which is similar to first order plots in that their. Half Life Equation Second Order.
From chemistnotes.com
Half life Formula Definition, and Wellderived equation Chemistry Notes Half Life Equation Second Order Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = 0.693 / k. T ½ = [a o] / 2k. However, second order reactions decrease at a much faster rate as the graph shows. For a second order reaction 2a products or a. For a zero order reaction a. Half Life Equation Second Order.
From www.numerade.com
the halflife equations for a firstorder and second Half Life Equation Second Order $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. T ½ = 0.693 / k. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: However, second order reactions decrease at a much. Half Life Equation Second Order.
From www.youtube.com
Second Order Integrated Rate Law and Half Life (Part 5) YouTube Half Life Equation Second Order For a first order reaction a products , rate = k [a]: $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a second order reaction 2a products or a. However, second order reactions decrease at a much faster rate as the graph shows. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time.. Half Life Equation Second Order.
From www.youtube.com
Understanding Halflife formulas with example. YouTube Half Life Equation Second Order T ½ = 0.693 / k. However, second order reactions decrease at a much faster rate as the graph shows. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: Time (\(t\)), which. Half Life Equation Second Order.
From www.slideserve.com
PPT Chemical or dynamics PowerPoint Presentation, free Half Life Equation Second Order For a first order reaction a products , rate = k [a]: Consider the reaction \(2a \rightarrow p\): For a second order reaction 2a products or a. T ½ = 0.693 / k. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a zero order reaction a products , rate. Half Life Equation Second Order.
From chemistnotes.com
Half life Formula Definition, and Wellderived equation Chemistry Notes Half Life Equation Second Order T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]: However, second order reactions decrease at a much faster rate as the graph shows. For a second order reaction 2a products or a. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. T ½ = [a o] / 2k. Consider the reaction \(2a \rightarrow. Half Life Equation Second Order.
From www.vrogue.co
How To Calculate Half Life For Second Order Reaction vrogue.co Half Life Equation Second Order However, second order reactions decrease at a much faster rate as the graph shows. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a second order reaction 2a products or a. T ½ = [a o] / 2k. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a zero order reaction. Half Life Equation Second Order.
From animalia-life.club
Half Life Chemistry Formula Half Life Equation Second Order T ½ = [a o] / 2k. However, second order reactions decrease at a much faster rate as the graph shows. Consider the reaction \(2a \rightarrow p\): For a zero order reaction a products , rate = k: T ½ = 0.693 / k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a first order reaction a products , rate. Half Life Equation Second Order.
From www.youtube.com
3integrated rate law 2nd order with halflife YouTube Half Life Equation Second Order Consider the reaction \(2a \rightarrow p\): For a second order reaction 2a products or a. T ½ = 0.693 / k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a zero order reaction a products , rate = k: However, second order reactions decrease at a much faster rate as the graph shows. T ½ = [a o] / 2k.. Half Life Equation Second Order.
From www.chegg.com
Solved Halflife for First and Second Order Reactions The Half Life Equation Second Order T ½ = 0.693 / k. For a zero order reaction a products , rate = k: However, second order reactions decrease at a much faster rate as the graph shows. For a first order reaction a products , rate = k [a]: Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with. Half Life Equation Second Order.
From www.youtube.com
Halflife of a secondorder reaction Chemistry Khan Half Life Equation Second Order T ½ = [a o] / 2k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Consider the reaction \(2a \rightarrow p\): For a second order reaction 2a products or a. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = 0.693 / k. For a first order reaction a products. Half Life Equation Second Order.
From righttecno.weebly.com
Half life of second order reaction righttecno Half Life Equation Second Order For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. T ½ = 0.693 / k. For a second order reaction 2a products or a. Time (\(t\)), which is similar to first order plots in that their slopes decrease to. Half Life Equation Second Order.
From www.youtube.com
Determine the halflife of a second order reaction YouTube Half Life Equation Second Order T ½ = 0.693 / k. However, second order reactions decrease at a much faster rate as the graph shows. Consider the reaction \(2a \rightarrow p\): For a first order reaction a products , rate = k [a]: $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Time (\(t\)), which is similar to first order plots in that their slopes decrease to. Half Life Equation Second Order.
From criticalthinking.cloud
how to solve a half life problem in chemistry Half Life Equation Second Order For a first order reaction a products , rate = k [a]: However, second order reactions decrease at a much faster rate as the graph shows. Consider the reaction \(2a \rightarrow p\): T ½ = [a o] / 2k. For a second order reaction 2a products or a. For a zero order reaction a products , rate = k: Time. Half Life Equation Second Order.
From www.youtube.com
Half life equation derivation Radioactivity YouTube Half Life Equation Second Order For a second order reaction 2a products or a. Consider the reaction \(2a \rightarrow p\): T ½ = 0.693 / k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a zero order reaction a products , rate = k: Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. However, second order. Half Life Equation Second Order.
From www.youtube.com
HalfLife Formula and Calculation Understanding Radioactive Decay Half Life Equation Second Order T ½ = [a o] / 2k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. For a second order reaction 2a products or a. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. Consider the. Half Life Equation Second Order.
From www.youtube.com
Integrated Rate Laws 1st and 2nd order linear and halflife equations Half Life Equation Second Order For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a zero order reaction a products , rate = k: Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a second order reaction 2a. Half Life Equation Second Order.
From www.slideshare.net
Lect w2 152 rate laws_alg Half Life Equation Second Order Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a zero order reaction a products , rate = k: Consider the reaction \(2a \rightarrow p\): T ½ = [a o] / 2k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a second order reaction 2a products or a. However, second. Half Life Equation Second Order.
From www.youtube.com
AP Chemistry 2 Integrated Rate Law & half life YouTube Half Life Equation Second Order However, second order reactions decrease at a much faster rate as the graph shows. For a first order reaction a products , rate = k [a]: Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = [a o] / 2k. For a second order reaction 2a products or a.. Half Life Equation Second Order.
From www.reddit.com
How to derive halflife equation r/chemistryhelp Half Life Equation Second Order T ½ = [a o] / 2k. For a second order reaction 2a products or a. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. Consider the reaction \(2a \rightarrow p\): However, second order. Half Life Equation Second Order.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Half Life Equation Second Order For a second order reaction 2a products or a. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: T ½ = 0.693 / k. Consider the reaction \(2a \rightarrow p\): $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. However, second. Half Life Equation Second Order.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Half Life Equation Second Order Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = 0.693 / k. T ½ = [a o] / 2k. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a second order reaction 2a products or a. However, second order reactions decrease at a much faster rate as the graph. Half Life Equation Second Order.
From www.chegg.com
Solved A secondorder reaction has a halflife of 12 s when Half Life Equation Second Order Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a first order reaction a products ,. Half Life Equation Second Order.
From ar.inspiredpencil.com
Half Life Formula Chemistry Half Life Equation Second Order For a zero order reaction a products , rate = k: For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. For a second order reaction 2a products or a. T ½ = 0.693 / k. Time (\(t\)), which is similar to first order plots in that their slopes decrease to. Half Life Equation Second Order.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation Second Order $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. However, second order reactions decrease at a much faster rate as the graph shows. For a second order reaction 2a products or a. For a zero order reaction a products , rate = k: For a first order reaction a products , rate = k [a]: Time (\(t\)), which is similar to first. Half Life Equation Second Order.
From www.studocu.com
Lesson 20 Halflife equations for 1st 2nd and 3rd order. 1 Lesson 20 Half Life Equation Second Order Consider the reaction \(2a \rightarrow p\): $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a second order reaction 2a products or a. However, second order reactions decrease at a much faster rate as the graph shows. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: T ½ = 0.693 / k.. Half Life Equation Second Order.
From www.youtube.com
How to Calculate HalfLife for Second Order Reactions YouTube Half Life Equation Second Order For a first order reaction a products , rate = k [a]: $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. Consider the reaction \(2a \rightarrow p\): For a second order reaction 2a products or a. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = 0.693 / k. For a. Half Life Equation Second Order.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free Half Life Equation Second Order For a first order reaction a products , rate = k [a]: $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. Consider the reaction \(2a \rightarrow p\): However, second order reactions decrease at a much faster rate as. Half Life Equation Second Order.
From jtpery.weebly.com
Half life of second order reaction jtpery Half Life Equation Second Order T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: $$\frac {1} { [a]_t}=kt+\frac {1} { [a]_0}$$. For a first order reaction a products , rate = k [a]: Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. T ½ = 0.693 /. Half Life Equation Second Order.
From www.slideserve.com
PPT Chapter 14 Chemical PowerPoint Presentation, free Half Life Equation Second Order For a first order reaction a products , rate = k [a]: For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. T ½ = [a o] / 2k. Consider the reaction \(2a \rightarrow p\): T ½ = 0.693 / k. However, second order reactions decrease at a much faster. Half Life Equation Second Order.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID5879103 Half Life Equation Second Order T ½ = [a o] / 2k. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a first order reaction a products , rate = k [a]: Consider the reaction \(2a \rightarrow p\): For a second order reaction 2a products or a. However, second order reactions decrease at a much. Half Life Equation Second Order.
From animalia-life.club
Half Life Chemistry Formula Half Life Equation Second Order Consider the reaction \(2a \rightarrow p\): T ½ = 0.693 / k. T ½ = [a o] / 2k. Time (\(t\)), which is similar to first order plots in that their slopes decrease to zero with time. For a first order reaction a products , rate = k [a]: However, second order reactions decrease at a much faster rate as. Half Life Equation Second Order.