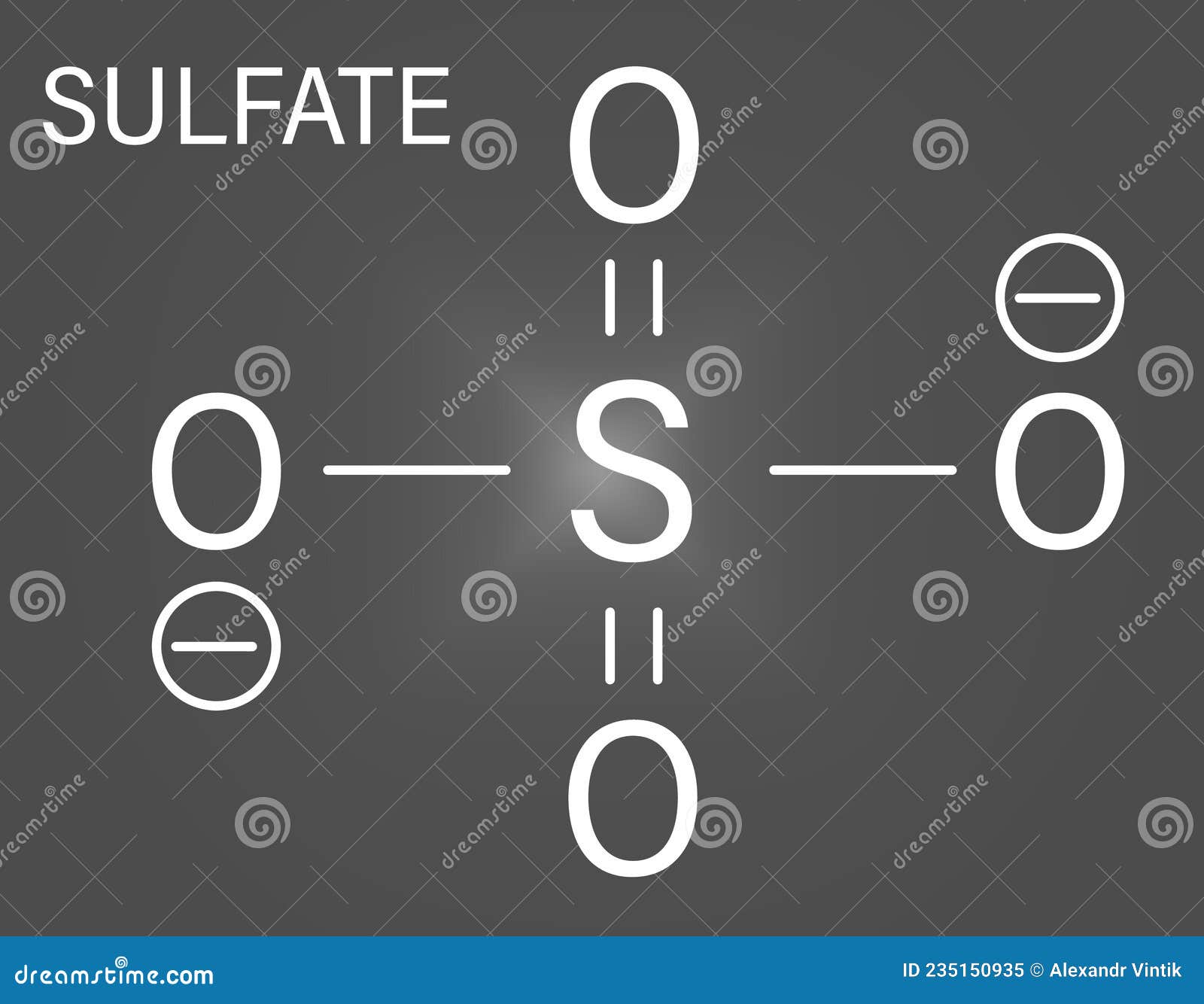
Aluminum Sulfate Anion Formula . The aluminum sulfate formula is. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. An example is aluminum sulfate, al 2 (so 4) 3. Determine formulas for simple ionic compounds. write the chemical formula for an ionic compound composed of each pair of ions. if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. By the end of this section, you will be able to: Sulfate is a polyatomic ion with formula (so4)2−. The sodium ion and the sulfur ion; Aluminum is a group 3a metal which. Lets take the ionic formula for calcium chloride. How do you write an ionic formula given an anion and a cation?
from www.dreamstime.com
Aluminum is a group 3a metal which. An example is aluminum sulfate, al 2 (so 4) 3. By the end of this section, you will be able to: The aluminum sulfate formula is. How do you write an ionic formula given an anion and a cation? Sulfate is a polyatomic ion with formula (so4)2−. Determine formulas for simple ionic compounds. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. The sodium ion and the sulfur ion; write the chemical formula for an ionic compound composed of each pair of ions.
Skeletal Formula of Sulfate Anion Molecule, Chemical Structure. Stock
Aluminum Sulfate Anion Formula How do you write an ionic formula given an anion and a cation? The sodium ion and the sulfur ion; How do you write an ionic formula given an anion and a cation? the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Aluminum is a group 3a metal which. An example is aluminum sulfate, al 2 (so 4) 3. By the end of this section, you will be able to: if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. Sulfate is a polyatomic ion with formula (so4)2−. Determine formulas for simple ionic compounds. The aluminum sulfate formula is. Lets take the ionic formula for calcium chloride. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. write the chemical formula for an ionic compound composed of each pair of ions.
From www.youtube.com
How to write the formula for aluminum sulfate YouTube Aluminum Sulfate Anion Formula if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. The aluminum sulfate formula is. An example is aluminum sulfate, al 2 (so 4) 3. Lets take the ionic formula for calcium chloride. aluminum sulfate is. Aluminum Sulfate Anion Formula.
From www.dreamstime.com
Skeletal Formula of Sulfate Anion, Chemical Structure. Stock Vector Aluminum Sulfate Anion Formula The sodium ion and the sulfur ion; Sulfate is a polyatomic ion with formula (so4)2−. Determine formulas for simple ionic compounds. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. How do you write an ionic formula given an anion and a cation? Aluminum is a group. Aluminum Sulfate Anion Formula.
From www.youtube.com
How to write chemical formula of Aluminium sulphate Chemical formula Aluminum Sulfate Anion Formula if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. An example is aluminum sulfate, al 2 (so 4) 3. By the end of this section, you will be able to: the lighter group 3a metals. Aluminum Sulfate Anion Formula.
From www.shutterstock.com
Sulfate Anion Molecular Skeletal Chemical Formula Stock Vector (Royalty Aluminum Sulfate Anion Formula Aluminum is a group 3a metal which. The aluminum sulfate formula is. Determine formulas for simple ionic compounds. An example is aluminum sulfate, al 2 (so 4) 3. write the chemical formula for an ionic compound composed of each pair of ions. How do you write an ionic formula given an anion and a cation? the lighter group. Aluminum Sulfate Anion Formula.
From www.sciencephoto.com
Sulfate anion chemical structure, illustration Stock Image F028 Aluminum Sulfate Anion Formula Lets take the ionic formula for calcium chloride. The sodium ion and the sulfur ion; The aluminum sulfate formula is. An example is aluminum sulfate, al 2 (so 4) 3. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. Aluminum is a group 3a metal which. . Aluminum Sulfate Anion Formula.
From www.dreamstime.com
Skeletal Formula of Sulfate Anion Molecule, Chemical Structure. Stock Aluminum Sulfate Anion Formula An example is aluminum sulfate, al 2 (so 4) 3. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. How do you write an ionic formula given an anion and a cation? Lets take the ionic formula for calcium chloride. Aluminum is a group 3a metal which.. Aluminum Sulfate Anion Formula.
From www.fishersci.ca
Aluminium sulfate, 99.999, (trace metal basis), extra pure, ACROS Aluminum Sulfate Anion Formula The aluminum sulfate formula is. Determine formulas for simple ionic compounds. How do you write an ionic formula given an anion and a cation? aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. the lighter group 3a metals (aluminum, galium and indium), along with scandium and. Aluminum Sulfate Anion Formula.
From clairekruwmunoz.blogspot.com
Give the Correct Formula for Aluminum Sulfate. ClairekruwMunoz Aluminum Sulfate Anion Formula The aluminum sulfate formula is. Sulfate is a polyatomic ion with formula (so4)2−. if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. By the end of this section, you will be able to: aluminum sulfate. Aluminum Sulfate Anion Formula.
From www.youtube.com
How to Write the Formula for Aluminum sulfate YouTube Aluminum Sulfate Anion Formula aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. Sulfate is a polyatomic ion with formula (so4)2−. The aluminum sulfate formula is. Aluminum is a group 3a metal which. Lets take the ionic formula for calcium chloride. The sodium ion and the sulfur ion; Determine formulas for. Aluminum Sulfate Anion Formula.
From aydin-blognorton.blogspot.com
What Is the Formula for Aluminum Sulfite Aluminum Sulfate Anion Formula Aluminum is a group 3a metal which. Sulfate is a polyatomic ion with formula (so4)2−. Lets take the ionic formula for calcium chloride. The aluminum sulfate formula is. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. if you need to add a subscript to a polyatomic. Aluminum Sulfate Anion Formula.
From www.drugs.com
Aluminum sulfate brand name list from Aluminum Sulfate Anion Formula Sulfate is a polyatomic ion with formula (so4)2−. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. write the chemical formula for an ionic compound composed of each pair of ions. Determine formulas for simple ionic compounds. if you need to add a subscript to a. Aluminum Sulfate Anion Formula.
From www.museoinclusivo.com
Exploring Aluminum Sulfate Formula Uses, Chemical Properties, and Aluminum Sulfate Anion Formula the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Aluminum is a group 3a metal which. Lets take the ionic formula for calcium chloride. By the end of this section, you will be able to: Determine formulas for simple ionic compounds. aluminum sulfate is an ionic compound. Aluminum Sulfate Anion Formula.
From www.alamy.com
Sulfate anion, chemical structure. Skeletal formula Stock Vector Image Aluminum Sulfate Anion Formula The sodium ion and the sulfur ion; The aluminum sulfate formula is. How do you write an ionic formula given an anion and a cation? aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. if you need to add a subscript to a polyatomic ion, enclose. Aluminum Sulfate Anion Formula.
From aluminumsulfatepitsukata.blogspot.com
Aluminum Sulfate Ionic Compound Formula For Aluminum Sulfate Aluminum Sulfate Anion Formula The aluminum sulfate formula is. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. Lets take the ionic formula for calcium chloride. The sodium ion and the sulfur ion; An example is aluminum sulfate, al 2 (so 4) 3. if you need to add a subscript. Aluminum Sulfate Anion Formula.
From www.pinterest.com
Aluminium sulfate Al2(SO4)3 Molecular Geometry Hybridization Aluminum Sulfate Anion Formula Lets take the ionic formula for calcium chloride. Determine formulas for simple ionic compounds. The aluminum sulfate formula is. if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. An example is aluminum sulfate, al 2 (so. Aluminum Sulfate Anion Formula.
From www.dreamstime.com
Skeletal Formula of Sulfate Anion Molecule, Chemical Structure. Stock Aluminum Sulfate Anion Formula write the chemical formula for an ionic compound composed of each pair of ions. Aluminum is a group 3a metal which. Sulfate is a polyatomic ion with formula (so4)2−. if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to. Aluminum Sulfate Anion Formula.
From www.dreamstime.com
Skeletal Formula of Sulfate Anion Molecule, Chemical Structure. Stock Aluminum Sulfate Anion Formula The sodium ion and the sulfur ion; Determine formulas for simple ionic compounds. Lets take the ionic formula for calcium chloride. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. Aluminum is a group 3a metal which. write the chemical formula for an ionic compound composed. Aluminum Sulfate Anion Formula.
From fyolwnaon.blob.core.windows.net
Aluminum Sulfate Decahydrate Chemical Formula at William Holbrook blog Aluminum Sulfate Anion Formula write the chemical formula for an ionic compound composed of each pair of ions. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. How do. Aluminum Sulfate Anion Formula.
From www.youtube.com
How to Write the Formula for Sodium aluminum sulfate YouTube Aluminum Sulfate Anion Formula By the end of this section, you will be able to: The aluminum sulfate formula is. How do you write an ionic formula given an anion and a cation? Aluminum is a group 3a metal which. Determine formulas for simple ionic compounds. Sulfate is a polyatomic ion with formula (so4)2−. An example is aluminum sulfate, al 2 (so 4) 3.. Aluminum Sulfate Anion Formula.
From www.dreamstime.com
Sulfate Anion, Chemical Structure. Skeletal Formula. Stock Illustration Aluminum Sulfate Anion Formula Determine formulas for simple ionic compounds. write the chemical formula for an ionic compound composed of each pair of ions. if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. The aluminum sulfate formula is. An. Aluminum Sulfate Anion Formula.
From h-o-m-e.org
Sulfate Ion's Charge Elucidated Aluminum Sulfate Anion Formula Aluminum is a group 3a metal which. write the chemical formula for an ionic compound composed of each pair of ions. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. Lets take the ionic formula for calcium chloride. the lighter group 3a metals (aluminum, galium. Aluminum Sulfate Anion Formula.
From www.shutterstock.com
Sulfate Anion Chemical Structure Skeletal Formula Vector có sẵn (miễn Aluminum Sulfate Anion Formula By the end of this section, you will be able to: Aluminum is a group 3a metal which. Lets take the ionic formula for calcium chloride. Sulfate is a polyatomic ion with formula (so4)2−. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. if you need. Aluminum Sulfate Anion Formula.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation ID2031508 Aluminum Sulfate Anion Formula the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. The aluminum sulfate formula is. Lets take the ionic formula for calcium chloride. The sodium ion and the sulfur ion; write the chemical formula for an ionic compound composed of each pair of ions. Sulfate is a polyatomic. Aluminum Sulfate Anion Formula.
From www.youtube.com
What is the chemical formula of Aluminium sulphate ? // chemical name Aluminum Sulfate Anion Formula How do you write an ionic formula given an anion and a cation? Determine formulas for simple ionic compounds. Sulfate is a polyatomic ion with formula (so4)2−. An example is aluminum sulfate, al 2 (so 4) 3. Aluminum is a group 3a metal which. By the end of this section, you will be able to: The aluminum sulfate formula is.. Aluminum Sulfate Anion Formula.
From www.sciencephoto.com
Sulfate anion chemical structure, illustration Stock Image F028 Aluminum Sulfate Anion Formula The sodium ion and the sulfur ion; The aluminum sulfate formula is. How do you write an ionic formula given an anion and a cation? if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. By the. Aluminum Sulfate Anion Formula.
From www.museoinclusivo.com
Exploring Aluminum Sulfate Formula Uses, Chemical Properties, and Aluminum Sulfate Anion Formula How do you write an ionic formula given an anion and a cation? if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. aluminum sulfate is an ionic compound that is made from two positively charged. Aluminum Sulfate Anion Formula.
From www.dreamstime.com
Skeletal Formula of Sulfate Anion, Chemical Structure. Stock Vector Aluminum Sulfate Anion Formula Determine formulas for simple ionic compounds. The aluminum sulfate formula is. if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations. Aluminum Sulfate Anion Formula.
From www.t3db.ca
T3DB Aluminium sulfate Aluminum Sulfate Anion Formula if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. The aluminum sulfate formula is. Determine formulas for simple ionic compounds. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Sulfate Anion Formula.
From www.museoinclusivo.com
What is the Chemical Formula for Aluminum Sulfate? Aluminum Profile Blog Aluminum Sulfate Anion Formula the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. The aluminum sulfate formula is. Aluminum is a group 3a metal which. Determine formulas for simple ionic. Aluminum Sulfate Anion Formula.
From www.alamy.com
Sulfate anion, chemical structure. Skeletal formula Stock Photo Alamy Aluminum Sulfate Anion Formula The sodium ion and the sulfur ion; aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. By the end of this section, you will be able to: Aluminum is a group 3a metal which. Sulfate is a polyatomic ion with formula (so4)2−. write the chemical formula. Aluminum Sulfate Anion Formula.
From www.alamy.com
Sulfate anion chemical structure, illustration Stock Photo Alamy Aluminum Sulfate Anion Formula if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. The sodium ion and the sulfur ion; aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged. Aluminum Sulfate Anion Formula.
From www.alamy.com
Sulfate anion, chemical structure. Skeletal formula Stock Vector Image Aluminum Sulfate Anion Formula How do you write an ionic formula given an anion and a cation? Determine formulas for simple ionic compounds. An example is aluminum sulfate, al 2 (so 4) 3. By the end of this section, you will be able to: aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged. Aluminum Sulfate Anion Formula.
From www.alamy.com
Sulfate anion (sulphate) molecule . Structural chemical formula and Aluminum Sulfate Anion Formula How do you write an ionic formula given an anion and a cation? aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. Determine formulas for simple ionic compounds. The aluminum sulfate formula is. By the end of this section, you will be able to: write the. Aluminum Sulfate Anion Formula.
From www.youtube.com
Equation for Al2(SO4)3 + H2O (Aluminum sulfate + Water) YouTube Aluminum Sulfate Anion Formula An example is aluminum sulfate, al 2 (so 4) 3. Determine formulas for simple ionic compounds. The aluminum sulfate formula is. aluminum sulfate is an ionic compound that is made from two positively charged aluminum cations and three negatively charged sulfate anions. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3. Aluminum Sulfate Anion Formula.
From www.pinterest.co.uk
anion Common anions, their names, formulas and the elements they are Aluminum Sulfate Anion Formula Sulfate is a polyatomic ion with formula (so4)2−. The sodium ion and the sulfur ion; Lets take the ionic formula for calcium chloride. if you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. write the chemical. Aluminum Sulfate Anion Formula.