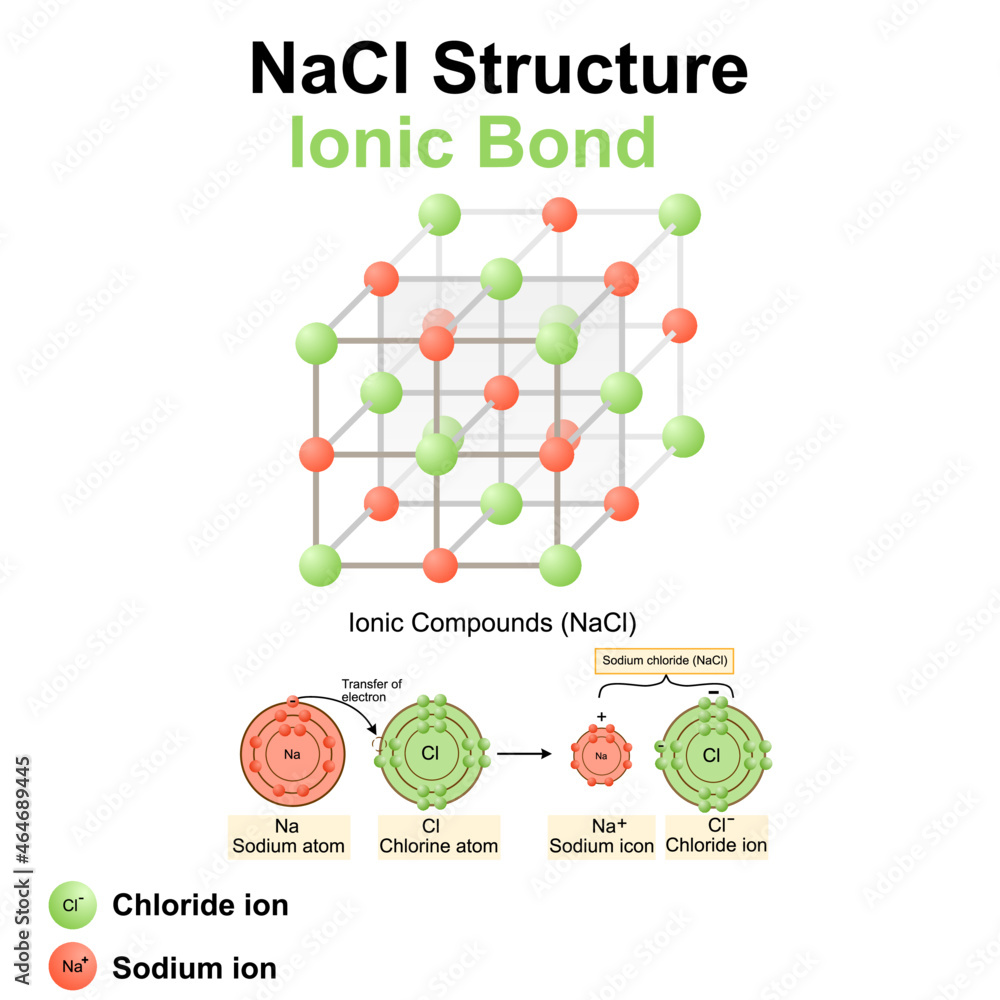
Chlorine Ionic Bond . Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. A bond’s strength describes how strongly each. A positive and negative ion attract each other and form an ionic bond. Opposite charges attract and like charges repel. Electrostatics explains why this happens: These oppositely charged ions attract each other to form ionic networks (or lattices). Students will look at animations and make drawings of the ionic bonding of sodium. Describe the energetics of ionic bond formation and breakage. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and.
from stock.adobe.com
Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. A positive and negative ion attract each other and form an ionic bond. Students will look at animations and make drawings of the ionic bonding of sodium. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Opposite charges attract and like charges repel. These oppositely charged ions attract each other to form ionic networks (or lattices). Electrostatics explains why this happens: Describe the energetics of ionic bond formation and breakage. A bond’s strength describes how strongly each. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties.
Sodium chloride (NaCl) molecule structure in 3d vector illustration
Chlorine Ionic Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Students will look at animations and make drawings of the ionic bonding of sodium. Electrostatics explains why this happens: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. A bond’s strength describes how strongly each. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. These oppositely charged ions attract each other to form ionic networks (or lattices). Opposite charges attract and like charges repel. A positive and negative ion attract each other and form an ionic bond. Describe the energetics of ionic bond formation and breakage.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Ionic Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. These oppositely charged ions attract each. Chlorine Ionic Bond.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Ionic Bond Electrostatics explains why this happens: Describe the energetics of ionic bond formation and breakage. Opposite charges attract and like charges repel. Students will look at animations and make drawings of the ionic bonding of sodium. A positive and negative ion attract each other and form an ionic bond. An atom of sodium (na) donates one of its electrons to an. Chlorine Ionic Bond.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Chlorine Ionic Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Electrostatics explains why this happens: A bond’s. Chlorine Ionic Bond.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Chlorine Ionic Bond Describe the energetics of ionic bond formation and breakage. These oppositely charged ions attract each other to form ionic networks (or lattices). A bond’s strength describes how strongly each. A positive and negative ion attract each other and form an ionic bond. Students will look at animations and make drawings of the ionic bonding of sodium. Learn about ionic and. Chlorine Ionic Bond.
From www.vrogue.co
Ionic Bond Vector Illustration Infographic Diagram Io vrogue.co Chlorine Ionic Bond Students will look at animations and make drawings of the ionic bonding of sodium. A positive and negative ion attract each other and form an ionic bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Describe the energetics of ionic bond formation and breakage. An atom of sodium. Chlorine Ionic Bond.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Ionic Bond Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Electrostatics explains why this happens: Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. A bond’s strength describes how strongly each. An atom of sodium (na) donates one of its. Chlorine Ionic Bond.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Ionic Bond An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. A bond’s strength describes how strongly each. A positive and negative ion attract each other and form an ionic bond. Students will look at animations and. Chlorine Ionic Bond.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Ionic Bond Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. A positive and negative ion attract each other and form an ionic bond. Opposite charges attract and like charges repel. Students will look at animations and make drawings of the ionic bonding of sodium. An atom of sodium (na) donates one. Chlorine Ionic Bond.
From www.dreamstime.com
Diagram To Show Ionic Bonding in Sodium Chloride Stock Illustration Chlorine Ionic Bond These oppositely charged ions attract each other to form ionic networks (or lattices). Describe the energetics of ionic bond formation and breakage. Opposite charges attract and like charges repel. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Electrostatics explains why this happens: A positive and negative ion attract each. Chlorine Ionic Bond.
From root469.blogspot.com
Ionic Bonding IGCSE Chemistry (0620) Best Notes Chlorine Ionic Bond Describe the energetics of ionic bond formation and breakage. A positive and negative ion attract each other and form an ionic bond. These oppositely charged ions attract each other to form ionic networks (or lattices). Electrostatics explains why this happens: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction,. Chlorine Ionic Bond.
From shiken.ai
Types of Chemical Bonds Chlorine Ionic Bond Electrostatics explains why this happens: Students will look at animations and make drawings of the ionic bonding of sodium. Opposite charges attract and like charges repel. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a.. Chlorine Ionic Bond.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Chlorine Ionic Bond A bond’s strength describes how strongly each. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a.. Chlorine Ionic Bond.
From stock.adobe.com
Sodium chloride (NaCl) molecule structure in 3d vector illustration Chlorine Ionic Bond Describe the energetics of ionic bond formation and breakage. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Electrostatics explains why this happens: Opposite charges attract and like charges repel. Chlorine has a far larger. Chlorine Ionic Bond.
From slideplayer.com
Chapter 22 Chemical Bonds Types of Bonds ppt download Chlorine Ionic Bond Opposite charges attract and like charges repel. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. A bond’s strength describes how strongly each. Describe the energetics of ionic bond formation and breakage. A positive and negative ion attract each other and form an ionic bond. Students will look at animations. Chlorine Ionic Bond.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine Ionic Bond Opposite charges attract and like charges repel. Students will look at animations and make drawings of the ionic bonding of sodium. Electrostatics explains why this happens: A bond’s strength describes how strongly each. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +). Chlorine Ionic Bond.
From derekcarrsavvy-chemist.blogspot.co.uk
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Ionic Bond Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. A positive and negative ion attract each other and form an ionic bond. A bond’s strength describes how strongly each. Describe the energetics of ionic bond formation and breakage. Electrostatics explains why this happens: Opposite charges attract and like charges. Chlorine Ionic Bond.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Chlorine Ionic Bond Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Opposite charges attract and like charges repel. An atom of sodium (na) donates one of its electrons to an atom of. Chlorine Ionic Bond.
From en.wikipedia.org
Ionic bonding Wikipedia Chlorine Ionic Bond Describe the energetics of ionic bond formation and breakage. Electrostatics explains why this happens: Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. A bond’s strength describes how strongly each. Opposite charges attract and like charges repel. Students will look at animations and make drawings of the ionic bonding. Chlorine Ionic Bond.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Ionic Bond A positive and negative ion attract each other and form an ionic bond. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Students will look at animations and make drawings of the ionic bonding of sodium. These oppositely charged ions attract each other to form ionic networks (or lattices). Describe. Chlorine Ionic Bond.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine Ionic Bond Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Opposite charges attract and like charges repel. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Electrostatics explains why this happens: Describe the energetics of ionic bond formation and breakage.. Chlorine Ionic Bond.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chlorine Ionic Bond Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. A bond’s strength describes how strongly. Chlorine Ionic Bond.
From www.savemyexams.com
Ionic Bonding AQA GCSE Chemistry Combined Science Revision Notes 2018 Chlorine Ionic Bond Students will look at animations and make drawings of the ionic bonding of sodium. Describe the energetics of ionic bond formation and breakage. Opposite charges attract and like charges repel. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Electrostatics explains why this happens: An atom of sodium (na) donates. Chlorine Ionic Bond.
From mammothmemory.net
Examples of ionic bonded substances such as sodium chloride Chlorine Ionic Bond Describe the energetics of ionic bond formation and breakage. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. A positive and negative ion attract each other and form an ionic bond. Opposite charges attract and like charges repel. Students will look at animations and make drawings of the ionic bonding. Chlorine Ionic Bond.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Ionic Bond A bond’s strength describes how strongly each. Opposite charges attract and like charges repel. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +). Chlorine Ionic Bond.
From www.slideserve.com
PPT Chapter 2 Life ’ s Chemical Basis PowerPoint Presentation, free Chlorine Ionic Bond A bond’s strength describes how strongly each. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Describe the energetics of ionic bond formation and breakage. Students will look at animations and make drawings of the ionic bonding of sodium. Electrostatics explains why this happens: These oppositely charged ions attract. Chlorine Ionic Bond.
From guidelistpstrapanning.z14.web.core.windows.net
Diagram Ionic Bond Chlorine Ionic Bond Opposite charges attract and like charges repel. A positive and negative ion attract each other and form an ionic bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Describe the energetics of ionic bond formation and breakage. Electrostatics explains why this happens: An atom of sodium (na) donates. Chlorine Ionic Bond.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6315263 Chlorine Ionic Bond Students will look at animations and make drawings of the ionic bonding of sodium. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na. Chlorine Ionic Bond.
From mammothmemory.net
Chemical bonding is about atoms achieving full outer shells Chlorine Ionic Bond Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. These oppositely charged ions attract each other to form ionic networks (or lattices). Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. A bond’s strength describes how strongly each. Students. Chlorine Ionic Bond.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Chlorine Ionic Bond Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Opposite charges attract and like charges repel. A positive and negative ion attract each other and form an ionic bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Electrostatics. Chlorine Ionic Bond.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Ionic Bond Opposite charges attract and like charges repel. A positive and negative ion attract each other and form an ionic bond. Electrostatics explains why this happens: Students will look at animations and make drawings of the ionic bonding of sodium. A bond’s strength describes how strongly each. These oppositely charged ions attract each other to form ionic networks (or lattices). Chlorine. Chlorine Ionic Bond.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Chlorine Ionic Bond These oppositely charged ions attract each other to form ionic networks (or lattices). A bond’s strength describes how strongly each. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Learn about ionic and covalent bonding, how metals react to form ionic compounds and how this affects their properties. Describe. Chlorine Ionic Bond.
From stock.adobe.com
Structure of sodium chloride (salt).NaCl model.Vector illustration Chlorine Ionic Bond A bond’s strength describes how strongly each. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Electrostatics explains why this happens: These oppositely charged ions attract each other to form ionic networks (or lattices). Students. Chlorine Ionic Bond.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Ionic Bond Students will look at animations and make drawings of the ionic bonding of sodium. Opposite charges attract and like charges repel. Electrostatics explains why this happens: Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. These oppositely charged ions attract each other to form ionic networks (or lattices). An. Chlorine Ionic Bond.
From fineartamerica.com
Ionic Bonding In Sodium Chloride, Artwork Photograph by Carlos Clarivan Chlorine Ionic Bond A positive and negative ion attract each other and form an ionic bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Describe the energetics of ionic bond formation and breakage. Electrostatics explains why this happens: An atom of sodium (na) donates one of its electrons to an atom. Chlorine Ionic Bond.
From tuitiontube.com
Ionic Bond and Ionic Bond Formation, Definition, Properties in Chlorine Ionic Bond Students will look at animations and make drawings of the ionic bonding of sodium. A positive and negative ion attract each other and form an ionic bond. Chlorine has a far larger electronegativity and so pulls the bonding electrons towards itself completely, thus gaining an electron and. Describe the energetics of ionic bond formation and breakage. A bond’s strength describes. Chlorine Ionic Bond.