Laboratory Criteria Definition . They can be utilized effectively at every. Clinical laboratories are healthcare facilities providing a wide range of. Role of osis with glp. this international standard specifies requirements for quality and competence in medical laboratories. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. It is a managerial concept covering. good laboratory practice (glp) is intended to promote the quality and validity of test data. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the.
from www.studocu.com
Role of osis with glp. It is a managerial concept covering. good laboratory practice (glp) is intended to promote the quality and validity of test data. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. Clinical laboratories are healthcare facilities providing a wide range of. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. They can be utilized effectively at every. this international standard specifies requirements for quality and competence in medical laboratories.
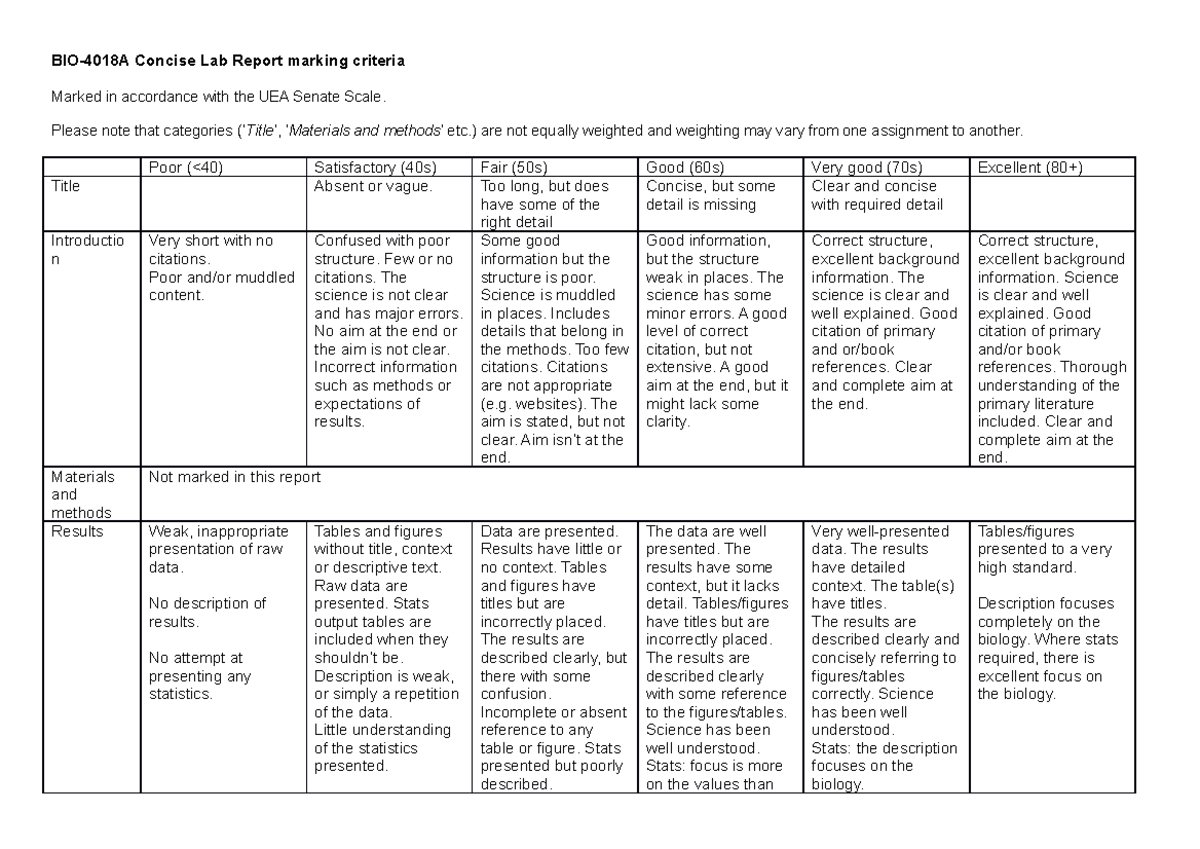
Marking criteria for 001 Concise Lab Report BIO4018A Concise Lab
Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. Clinical laboratories are healthcare facilities providing a wide range of. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. good laboratory practice (glp) is intended to promote the quality and validity of test data. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. this international standard specifies requirements for quality and competence in medical laboratories. Role of osis with glp. It is a managerial concept covering. They can be utilized effectively at every.
From dokumen.tips
(PDF) Towards Definition, Clinical and Laboratory Criteria, and · a Laboratory Criteria Definition laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. good laboratory practice (glp) is intended to promote the quality and validity of test data. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. the principles of good laboratory practice (glp) define a. Laboratory Criteria Definition.
From haembase.com
MGUS — HaemBase Laboratory Criteria Definition good laboratory practice (glp) is intended to promote the quality and validity of test data. this international standard specifies requirements for quality and competence in medical laboratories. They can be utilized effectively at every. Role of osis with glp. It is a managerial concept covering. Clinical laboratories are healthcare facilities providing a wide range of. the principles. Laboratory Criteria Definition.
From www.cmaj.ca
Updated SARS case definition using laboratory criteria CMAJ Laboratory Criteria Definition They can be utilized effectively at every. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. this international standard specifies requirements for quality and competence in medical laboratories. good laboratory practice (glp) is intended to promote the quality and validity of test data. Clinical laboratories are healthcare facilities providing a wide range. Laboratory Criteria Definition.
From www.slideserve.com
PPT Classification criteria for APS Laboratory Criteria PowerPoint Laboratory Criteria Definition Role of osis with glp. It is a managerial concept covering. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. Clinical laboratories are healthcare facilities providing a wide range of. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. this international standard specifies. Laboratory Criteria Definition.
From www.researchgate.net
(PDF) Updated SARS case definition using laboratory criteria Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. It is a managerial concept covering. good laboratory practice (glp) is intended to promote the quality and validity of test data. Role of osis with glp. They can be utilized effectively. Laboratory Criteria Definition.
From www.hepatitisc.uw.edu
Core Concepts HCV Epidemiology in the United States Screening and Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. Clinical laboratories are healthcare facilities providing a wide range of. It is a managerial concept covering. we describe eleven core elements that constitute the gclp standards with the objective of filling. Laboratory Criteria Definition.
From www.studocu.com
Marking criteria for 001 Concise Lab Report BIO4018A Concise Lab Laboratory Criteria Definition Role of osis with glp. good laboratory practice (glp) is intended to promote the quality and validity of test data. It is a managerial concept covering. They can be utilized effectively at every. this international standard specifies requirements for quality and competence in medical laboratories. Clinical laboratories are healthcare facilities providing a wide range of. we describe. Laboratory Criteria Definition.
From www.slideserve.com
PPT Case C onference PowerPoint Presentation, free download ID2344829 Laboratory Criteria Definition the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. They can be utilized effectively at every. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. It is a managerial concept covering. this international standard specifies requirements for quality and competence. Laboratory Criteria Definition.
From www.oecd-ilibrary.org
4. Understanding the six criteria Definitions, elements for analysis Laboratory Criteria Definition They can be utilized effectively at every. Clinical laboratories are healthcare facilities providing a wide range of. It is a managerial concept covering. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. this international standard specifies requirements for quality and competence in medical laboratories. Role of osis with glp. we describe eleven. Laboratory Criteria Definition.
From studylib.net
Measurements Lab Requirements pt 1 Laboratory Criteria Definition They can be utilized effectively at every. Role of osis with glp. Clinical laboratories are healthcare facilities providing a wide range of. good laboratory practice (glp) is intended to promote the quality and validity of test data. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. the principles. Laboratory Criteria Definition.
From grade7sciencebinus.blogspot.com
MYP Science Grade 7 Assessment Guidelines Criteria B and C Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. It is a managerial concept covering. Clinical laboratories are healthcare facilities providing a wide range of. They can be utilized effectively at every. Role of osis with glp. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system. Laboratory Criteria Definition.
From www.slideserve.com
PPT Nutrition Screening and Assessment PowerPoint Presentation, free Laboratory Criteria Definition the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. It is a managerial concept covering. They can be utilized effectively at every. this international standard specifies requirements for quality and competence in medical laboratories. Role of osis with glp. laboratory quality can be defined as. Laboratory Criteria Definition.
From www.slideserve.com
PPT Classification criteria for APS Laboratory Criteria PowerPoint Laboratory Criteria Definition They can be utilized effectively at every. this international standard specifies requirements for quality and competence in medical laboratories. Clinical laboratories are healthcare facilities providing a wide range of. good laboratory practice (glp) is intended to promote the quality and validity of test data. the principles of good laboratory practice (glp) define a set of rules and. Laboratory Criteria Definition.
From www.studocu.com
PYB102 Laboratory Report Marking Criteria Sheet 2021 Grade 7 6 5 4 3 Laboratory Criteria Definition we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. Clinical laboratories are healthcare facilities providing a wide range of. Role of osis with glp. this international standard specifies requirements for quality and competence in medical laboratories. good laboratory practice (glp) is intended to promote the quality and validity. Laboratory Criteria Definition.
From www.researchgate.net
Laboratory criteria for case definition of autochthonous Laboratory Criteria Definition the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. It is a managerial concept covering. They can be utilized effectively at every. Clinical laboratories are healthcare facilities providing a wide range of. we describe eleven core elements that constitute the gclp standards with the objective of. Laboratory Criteria Definition.
From www.mdedge.com
Diagnosing and Classifying Anemia in Adult Primary Care Clinician Reviews Laboratory Criteria Definition we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. this international standard specifies requirements for quality and competence in medical laboratories. Role of osis with glp. It is a managerial concept covering. good laboratory practice (glp) is intended to promote the quality and validity of test data. . Laboratory Criteria Definition.
From www.studocu.com
Practical Lab Marking Criteria Practical Lab Marking Criteria Laboratory Criteria Definition Clinical laboratories are healthcare facilities providing a wide range of. Role of osis with glp. They can be utilized effectively at every. this international standard specifies requirements for quality and competence in medical laboratories. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. the principles of good laboratory practice (glp) define a. Laboratory Criteria Definition.
From www.slideserve.com
PPT Instructions for users PowerPoint Presentation, free download Laboratory Criteria Definition Role of osis with glp. good laboratory practice (glp) is intended to promote the quality and validity of test data. They can be utilized effectively at every. Clinical laboratories are healthcare facilities providing a wide range of. It is a managerial concept covering. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. . Laboratory Criteria Definition.
From www.hepatitisc.uw.edu
Core Concepts HCV Epidemiology in the United States Screening and Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. Clinical laboratories are healthcare facilities providing a wide range of. good laboratory practice (glp) is intended to promote the quality and validity of test data. They can be utilized effectively at every. It is a managerial concept covering. Role of osis with glp. we describe. Laboratory Criteria Definition.
From www.slideserve.com
PPT WNV Human Case Investigation and Reporting PowerPoint Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. It is a managerial concept covering. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. Clinical laboratories are healthcare facilities providing a wide range of. we describe eleven core elements that constitute the gclp standards with the objective of filling. Laboratory Criteria Definition.
From www.researchgate.net
(PDF) Towards definition, clinical and laboratory criteria, and a Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. They can be utilized effectively at every. good laboratory practice (glp) is intended to promote the quality and validity of test data. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. Role of osis with glp. the principles of. Laboratory Criteria Definition.
From www.researchgate.net
Comparison of laboratory criteria for APS. Download Table Laboratory Criteria Definition the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. good laboratory practice (glp) is intended to promote the quality and validity of test data. Clinical laboratories are healthcare facilities providing a wide range of. this international standard specifies requirements for quality and competence in medical. Laboratory Criteria Definition.
From www.altexsoft.com
Complete Guide to Laboratory Information Management Systems (LIMS Laboratory Criteria Definition good laboratory practice (glp) is intended to promote the quality and validity of test data. Clinical laboratories are healthcare facilities providing a wide range of. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. Role of osis with glp. laboratory quality can be defined as. Laboratory Criteria Definition.
From www.gdwaldner.com
Waldner Magazine Bio Safety Labs Classification Laboratory Criteria Definition laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. this international standard specifies requirements for quality and competence in medical laboratories. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. Role of osis with glp. good laboratory practice (glp) is intended to. Laboratory Criteria Definition.
From academy.pubs.asha.org
Tips for Setting Up Your Lab ASHA Journals Academy Laboratory Criteria Definition laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. good laboratory practice (glp) is intended to promote the quality and validity of test data. this international standard specifies requirements for quality and competence in medical laboratories. we describe eleven core elements that constitute the gclp standards with the objective of filling. Laboratory Criteria Definition.
From cenmedonline.com
The Ultimate Laboratory Checklist Cenmed Enterprises Laboratory Criteria Definition It is a managerial concept covering. this international standard specifies requirements for quality and competence in medical laboratories. good laboratory practice (glp) is intended to promote the quality and validity of test data. Clinical laboratories are healthcare facilities providing a wide range of. the principles of good laboratory practice (glp) define a set of rules and criteria. Laboratory Criteria Definition.
From www.hiv.uw.edu
Core Concepts Initial Evaluation Basic HIV Primary Care National Laboratory Criteria Definition laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. It is a managerial concept covering. this international standard specifies requirements for quality and competence in medical laboratories. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. we describe eleven. Laboratory Criteria Definition.
From www.scribd.com
Lab Report Grading Rubric PDF Experiment Hypothesis Laboratory Criteria Definition we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. good laboratory practice (glp) is intended to promote the quality and validity of test data. Role of osis with glp. the principles of good. Laboratory Criteria Definition.
From dxoatrmoc.blob.core.windows.net
Calibration And Quality Control In Laboratory Pdf at Marcia Snyder blog Laboratory Criteria Definition this international standard specifies requirements for quality and competence in medical laboratories. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. Clinical laboratories are healthcare facilities providing a wide range of. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. good laboratory. Laboratory Criteria Definition.
From organicindiatoday.com
40 Lab Report Templates & Format Examples ᐅ TemplateLab Organic Articles Laboratory Criteria Definition Clinical laboratories are healthcare facilities providing a wide range of. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. good laboratory practice (glp) is intended to promote the quality and validity of test data.. Laboratory Criteria Definition.
From studylib.net
Grading Criteria Guide for lab experiment report 03202013.doc Laboratory Criteria Definition Role of osis with glp. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. Clinical laboratories are healthcare facilities providing a wide range of. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. They can be utilized. Laboratory Criteria Definition.
From havenortho.com
Basic Lab Requirements Laboratory Criteria Definition They can be utilized effectively at every. good laboratory practice (glp) is intended to promote the quality and validity of test data. this international standard specifies requirements for quality and competence in medical laboratories. It is a managerial concept covering. Clinical laboratories are healthcare facilities providing a wide range of. laboratory quality can be defined as accuracy,. Laboratory Criteria Definition.
From studylib.net
CLINICAL LABORATORY TESTS REFERENCE VALUES Laboratory Criteria Definition Clinical laboratories are healthcare facilities providing a wide range of. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. this international standard specifies requirements for quality and competence in medical laboratories. It is a managerial concept covering. we describe eleven core elements that constitute the. Laboratory Criteria Definition.
From www.the-hospitalist.org
How Is SIADH Diagnosed and Managed? Page 2 of 4 The Hospitalist Laboratory Criteria Definition Role of osis with glp. laboratory quality can be defined as accuracy, reliability and timeliness of reported test results. They can be utilized effectively at every. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. It is a managerial concept covering. Clinical laboratories are healthcare facilities. Laboratory Criteria Definition.
From www.slideserve.com
PPT Classification criteria for APS Laboratory Criteria PowerPoint Laboratory Criteria Definition It is a managerial concept covering. we describe eleven core elements that constitute the gclp standards with the objective of filling a gap for. They can be utilized effectively at every. Role of osis with glp. this international standard specifies requirements for quality and competence in medical laboratories. Clinical laboratories are healthcare facilities providing a wide range of.. Laboratory Criteria Definition.