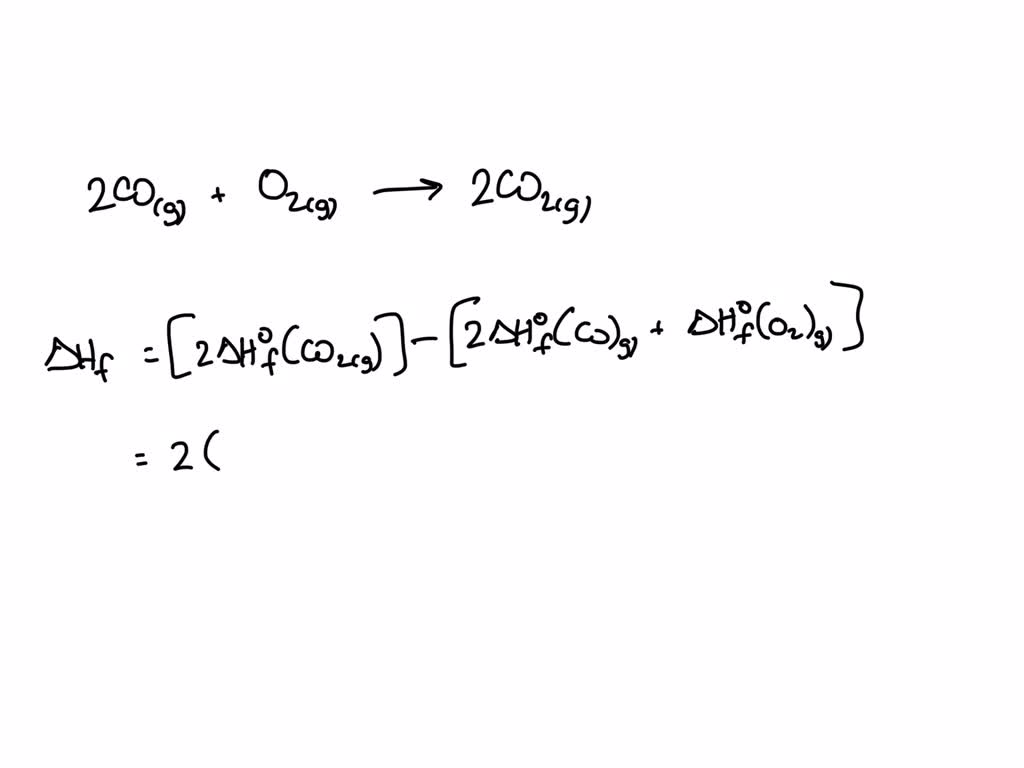Standard Enthalpy Of Formation Vs Reaction . yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. standard enthalpies of formation. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or.
from www.numerade.com
The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. standard enthalpies of formation. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the.
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g
Standard Enthalpy Of Formation Vs Reaction yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the.
From dxogyycoh.blob.core.windows.net
Standard Enthalpy Of Formation Barium Hydroxide at Alice Connor blog Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpies of formation. the enthalpy of formation is the enthalpy. Standard Enthalpy Of Formation Vs Reaction.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. the standard enthalpy of formation is given the symbol δfho δ f. Standard Enthalpy Of Formation Vs Reaction.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Vs Reaction The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly. Standard Enthalpy Of Formation Vs Reaction.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Vs Reaction yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of. Standard Enthalpy Of Formation Vs Reaction.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Vs Reaction the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. standard enthalpies of formation. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is. Standard Enthalpy Of Formation Vs Reaction.
From learningy5li0r.z21.web.core.windows.net
How To Determine The Heat Of Formation Standard Enthalpy Of Formation Vs Reaction standard enthalpies of formation. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. yes, the standard enthalpy of reaction. Standard Enthalpy Of Formation Vs Reaction.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. standard enthalpies of formation. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. The magnitude of δ h for a reaction. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Vs Reaction standard enthalpies of formation. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. the standard enthalpy of formation is a measure of the energy released or. Standard Enthalpy Of Formation Vs Reaction.
From www.msjchem.com
Topic 5 Energetics MSJChem Tutorial videos for IB Chemistry Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. standard enthalpies of formation. the standard enthalpy of formation is a. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Vs Reaction yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the.. Standard Enthalpy Of Formation Vs Reaction.
From www.slideserve.com
PPT Enthalpy An introduction to Chemical Thermodynamics PowerPoint Standard Enthalpy Of Formation Vs Reaction standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
6.6 Standard Enthalpy of Formation and Reaction YouTube Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. the standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation Vs Reaction.
From people.chem.umass.edu
to Adobe GoLive 6 Standard Enthalpy Of Formation Vs Reaction the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
Enthalpy of formation Thermodynamics AP Chemistry Khan Academy Standard Enthalpy Of Formation Vs Reaction The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. standard enthalpies of formation. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$). Standard Enthalpy Of Formation Vs Reaction.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Vs Reaction The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the enthalpy of formation is the enthalpy difference between the product and. Standard Enthalpy Of Formation Vs Reaction.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Vs Reaction the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpies of formation. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is. Standard Enthalpy Of Formation Vs Reaction.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Standard Enthalpy Of Formation Vs Reaction yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
6.9 Determining Enthalpies of Reaction from Standard Enthalpies of Standard Enthalpy Of Formation Vs Reaction the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpies of formation. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. the enthalpy of formation is the enthalpy difference between the product. Standard Enthalpy Of Formation Vs Reaction.
From www.numerade.com
SOLVED Calculate the standard Gibbs energy of the reaction CO(g Standard Enthalpy Of Formation Vs Reaction the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. The magnitude of δ h for a reaction depends on the physical states of the reactants and. Standard Enthalpy Of Formation Vs Reaction.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Vs Reaction standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. standard enthalpy of formation is the enthalpy. Standard Enthalpy Of Formation Vs Reaction.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Enthalpy Of Formation Vs Reaction standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. standard enthalpies of formation. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. a standard enthalpy of formation $δh°_f$ is an enthalpy change for. Standard Enthalpy Of Formation Vs Reaction.
From www.chegg.com
Solved The standard enthalpy (or heat) of reaction, delta H, Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the enthalpy of formation is the enthalpy difference between the product and. Standard Enthalpy Of Formation Vs Reaction.
From www.vrogue.co
Physical Chemistry 15 Enthalpy Change And Enthalpy Pr vrogue.co Standard Enthalpy Of Formation Vs Reaction The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent.. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Vs Reaction the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. the standard enthalpy of formation is a measure of the energy released. Standard Enthalpy Of Formation Vs Reaction.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation Vs Reaction the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs. Standard Enthalpy Of Formation Vs Reaction.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Vs Reaction the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates that the. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a. Standard Enthalpy Of Formation Vs Reaction.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Vs Reaction a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the enthalpy of formation is the enthalpy difference between the product and. Standard Enthalpy Of Formation Vs Reaction.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Standard Enthalpy Of Formation Vs Reaction standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. standard enthalpy of formation is the enthalpy change when one mole of any compound. Standard Enthalpy Of Formation Vs Reaction.
From www.thesciencehive.co.uk
Enthalpy Changes — the science hive Standard Enthalpy Of Formation Vs Reaction the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. The magnitude of δ h for a reaction depends on the physical states of the reactants and the products. Standard Enthalpy Of Formation Vs Reaction.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Of Formation Vs Reaction The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. the enthalpy of formation is the enthalpy difference between the product and the elements of formation in their standard state, while the enthalpy. the standard enthalpy of formation is given the symbol δfho δ f. Standard Enthalpy Of Formation Vs Reaction.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Vs Reaction The magnitude of δ h for a reaction depends on the physical states of the reactants and the products (gas, liquid, solid, or. standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is. Standard Enthalpy Of Formation Vs Reaction.
From chemistry.stackexchange.com
thermodynamics Calculating Enthalpy of formation versus Calculating Standard Enthalpy Of Formation Vs Reaction standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. The magnitude of δ h for a reaction. Standard Enthalpy Of Formation Vs Reaction.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Vs Reaction yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Standard Enthalpy Of Formation Vs Reaction.
From www.youtube.com
How to Calculate the Standard Enthalpy of a Reaction from the Standard Standard Enthalpy Of Formation Vs Reaction yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system. standard enthalpy of formation is the enthalpy change when one mole of any compound is formed from its constituent. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.. Standard Enthalpy Of Formation Vs Reaction.