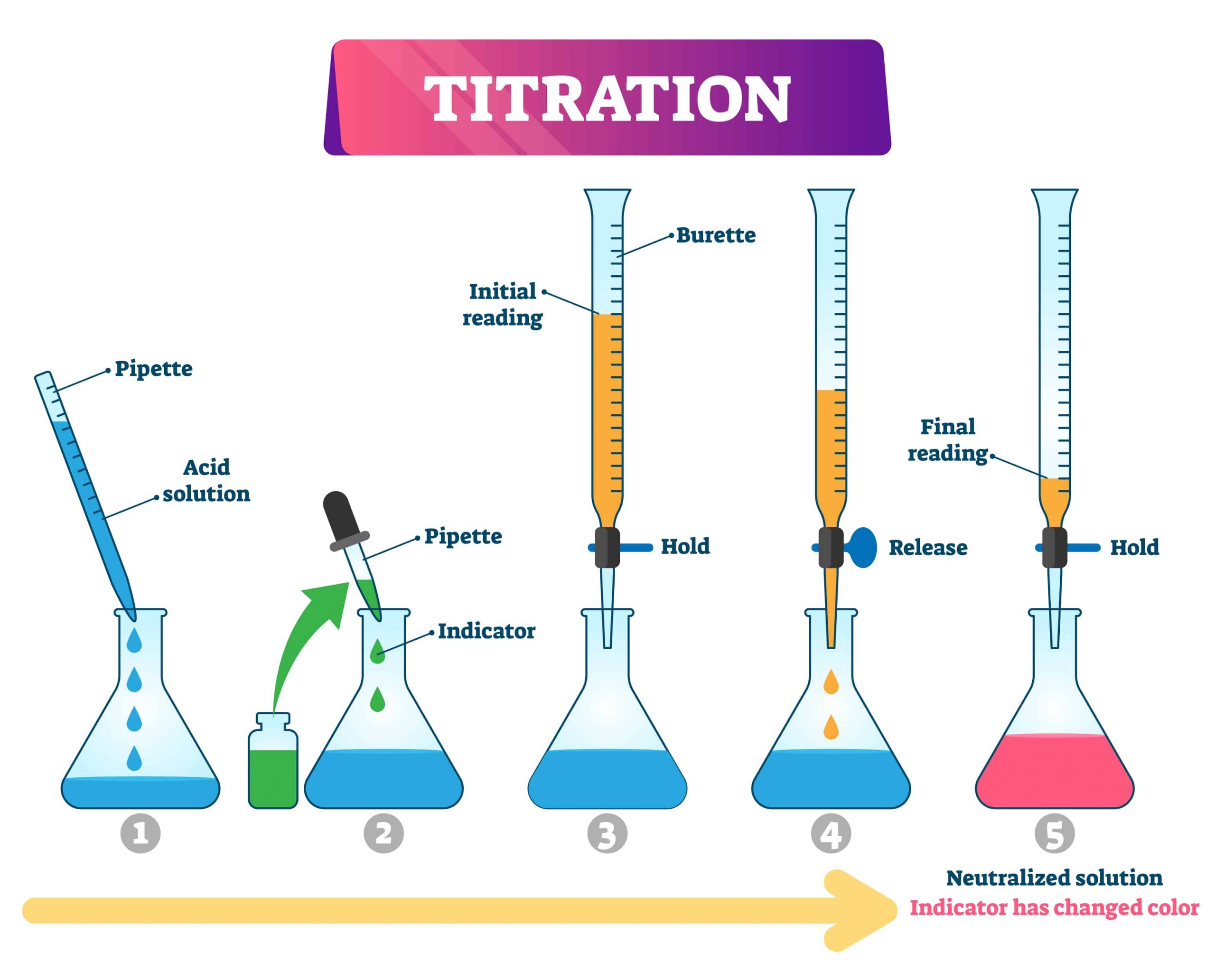
Discussion Of Titration . In an indicator based titration you add another chemical that changes color at the ph equal to the. There are two basic types of acid base titrations, indicator and potentiometric. A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Close the tap when you see a colour change. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). Open the burette tap and let down solution b into the conical flask while you swirl the flask.
from www.reagent.co.uk
Close the tap when you see a colour change. The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). There are two basic types of acid base titrations, indicator and potentiometric. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. In an indicator based titration you add another chemical that changes color at the ph equal to the. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown.
How is Titration Used in the Pharmaceutical Industry?
Discussion Of Titration Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. There are two basic types of acid base titrations, indicator and potentiometric. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. Close the tap when you see a colour change. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. In an indicator based titration you add another chemical that changes color at the ph equal to the. Open the burette tap and let down solution b into the conical flask while you swirl the flask. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion.
From www.slideshare.net
Acid base titration Discussion Of Titration A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Note the start point on the burette (it might not be 0.00 cm 3) in a results. Discussion Of Titration.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Discussion Of Titration A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample. Discussion Of Titration.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Discussion Of Titration Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). In an indicator based titration you add another chemical that changes color at the ph equal to. Discussion Of Titration.
From www.slideshare.net
Titration report Discussion Of Titration First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). There are two basic types of acid base titrations, indicator and potentiometric. A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Open the burette. Discussion Of Titration.
From www.scribd.com
Titration PDF Titration Chemistry Discussion Of Titration Close the tap when you see a colour change. Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. Open the burette. Discussion Of Titration.
From www.studypool.com
SOLUTION Conductometric titration II lab report Studypool Discussion Of Titration Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. The power. Discussion Of Titration.
From www.science-revision.co.uk
Titrations Discussion Of Titration In an indicator based titration you add another chemical that changes color at the ph equal to the. The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. Titration is the slow addition of one solution of a known. Discussion Of Titration.
From snipe.fm
😂 Titration lab report discussion. Acid base TITRATION EXPERIMENT lab Discussion Of Titration Note the start point on the burette (it might not be 0.00 cm 3) in a results table. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). In an indicator based titration you add another chemical that changes color at the ph equal. Discussion Of Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Discussion Of Titration Note the start point on the burette (it might not be 0.00 cm 3) in a results table. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Open the burette tap and let down solution b into the conical flask while you swirl the flask.. Discussion Of Titration.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Discussion Of Titration A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is an experiment where a volume of a solution of known concentration is added to. Discussion Of Titration.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Discussion Of Titration A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. There are two basic types of acid base titrations, indicator and potentiometric. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3. Discussion Of Titration.
From studylib.net
Laboratory 5 Titrations Introduction Discussion Discussion Of Titration Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. Open the burette tap and let down solution b into the conical flask while you swirl the flask. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to.. Discussion Of Titration.
From www.thecannabiscommunity.org
What Is Titration? Benefits, Tips and How To Do It Right Discussion Of Titration First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). There are two basic types of acid base titrations, indicator and potentiometric. Open the burette tap and let down solution b into the conical flask while you swirl the flask. Titration, process of chemical. Discussion Of Titration.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Discussion Of Titration There are two basic types of acid base titrations, indicator and potentiometric. Close the tap when you see a colour change. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite,. Discussion Of Titration.
From www.biorender.com
AcidBase Titration BioRender Science Templates Discussion Of Titration The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. Open the burette tap and let down solution b into the conical flask while you swirl the flask. There are two basic types of acid base titrations, indicator and. Discussion Of Titration.
From www.pathwaystochemistry.com
Titrations Introduction Pathways to Chemistry Discussion Of Titration Close the tap when you see a colour change. There are two basic types of acid base titrations, indicator and potentiometric. The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. Titration is a laboratory technique used to determine. Discussion Of Titration.
From chem4three.blogspot.co.uk
CHEMISTRY 11 TITRATIONS Discussion Of Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. Open the burette tap and let down solution b into the conical flask while you swirl the flask.. Discussion Of Titration.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Discussion Of Titration Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. There are two basic types of acid base titrations, indicator and potentiometric. Close the tap when you see a colour change. Open the burette tap and let down solution b into the conical flask while you swirl the flask. A titration is one. Discussion Of Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Discussion Of Titration Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The power of the process is its simplicity, as there is a visual indication, a color change, that. Discussion Of Titration.
From www.analytica-world.com
Titration Handbook Theory and Practice of Titration A guide to Discussion Of Titration There are two basic types of acid base titrations, indicator and potentiometric. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Titration is the slow addition. Discussion Of Titration.
From jagomart.net
Aspirin Tablets Titration Discussion Of Titration A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. Close the tap when you see a colour change. A titration is an experiment where a volume of a solution of known concentration. Discussion Of Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Discussion Of Titration In an indicator based titration you add another chemical that changes color at the ph equal to the. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. The. Discussion Of Titration.
From resource.studiaacademy.com
Acids, Alkalis and Titrations Studia Academy Resources Discussion Of Titration There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). The power of the. Discussion Of Titration.
From exyouyqrf.blob.core.windows.net
Titration Apparatus And Uses at Nicole Fain blog Discussion Of Titration A titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Open the burette tap and let down solution b into the conical flask while you swirl the flask. Close the tap when you see a colour change. Titration is a laboratory technique used to determine. Discussion Of Titration.
From fity.club
Titration Techniques Chemistry Tutorial Discussion Of Titration Note the start point on the burette (it might not be 0.00 cm 3) in a results table. In an indicator based titration you add another chemical that changes color at the ph equal to the. Close the tap when you see a colour change. Titration, process of chemical analysis in which the quantity of some constituent of a sample. Discussion Of Titration.
From www.scribd.com
Week 4. Titration Titration Chemistry Discussion Of Titration First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes color at the ph equal to the. A titration is one. Discussion Of Titration.
From exypvoihf.blob.core.windows.net
Discussion For Titration at Jacqueline Demaio blog Discussion Of Titration The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Close the tap when you see a colour change. First,. Discussion Of Titration.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Discussion Of Titration The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. In an indicator based titration you add another chemical that changes color at the ph equal to the. A titration is an experiment where a volume of a solution. Discussion Of Titration.
From gioknvtfx.blob.core.windows.net
Lab Report On Acid Base Titration at Linda Crocker blog Discussion Of Titration Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. First, do a rough titration, followed by as many accurate titrations as you need to get concordant results (results within 0.10 cm 3 of each other). In an indicator based titration you add another chemical that changes color at the ph equal to. Discussion Of Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Discussion Of Titration A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. There are two basic types of acid base titrations, indicator and potentiometric. Titration is the slow addition of one solution of a known. Discussion Of Titration.
From www.studypool.com
SOLUTION Acid base titration chemistry discussion Studypool Discussion Of Titration Open the burette tap and let down solution b into the conical flask while you swirl the flask. Close the tap when you see a colour change. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the. Discussion Of Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Discussion Of Titration Titration is a laboratory technique used to determine the concentration of an unknown chemical in a solution. A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Open the burette tap and let down solution b into the conical flask while you swirl the flask. Note the start point on the. Discussion Of Titration.
From www.youtube.com
Acid Base Titration Lecture 3 Chemistry Practicals Fsc 1st Year Discussion Of Titration In an indicator based titration you add another chemical that changes color at the ph equal to the. The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. There are two basic types of acid base titrations, indicator and. Discussion Of Titration.
From tukioka-clinic.com
😍 Titration lab discussion. Discussion exp 1. 20190123 Discussion Of Titration Note the start point on the burette (it might not be 0.00 cm 3) in a results table. In an indicator based titration you add another chemical that changes color at the ph equal to the. A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. Close the tap when you. Discussion Of Titration.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Discussion Of Titration Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of another substance with which the desired constituent reacts in a definite, known proportion. Note the start point on the burette (it might not be 0.00 cm 3) in a results table. In. Discussion Of Titration.