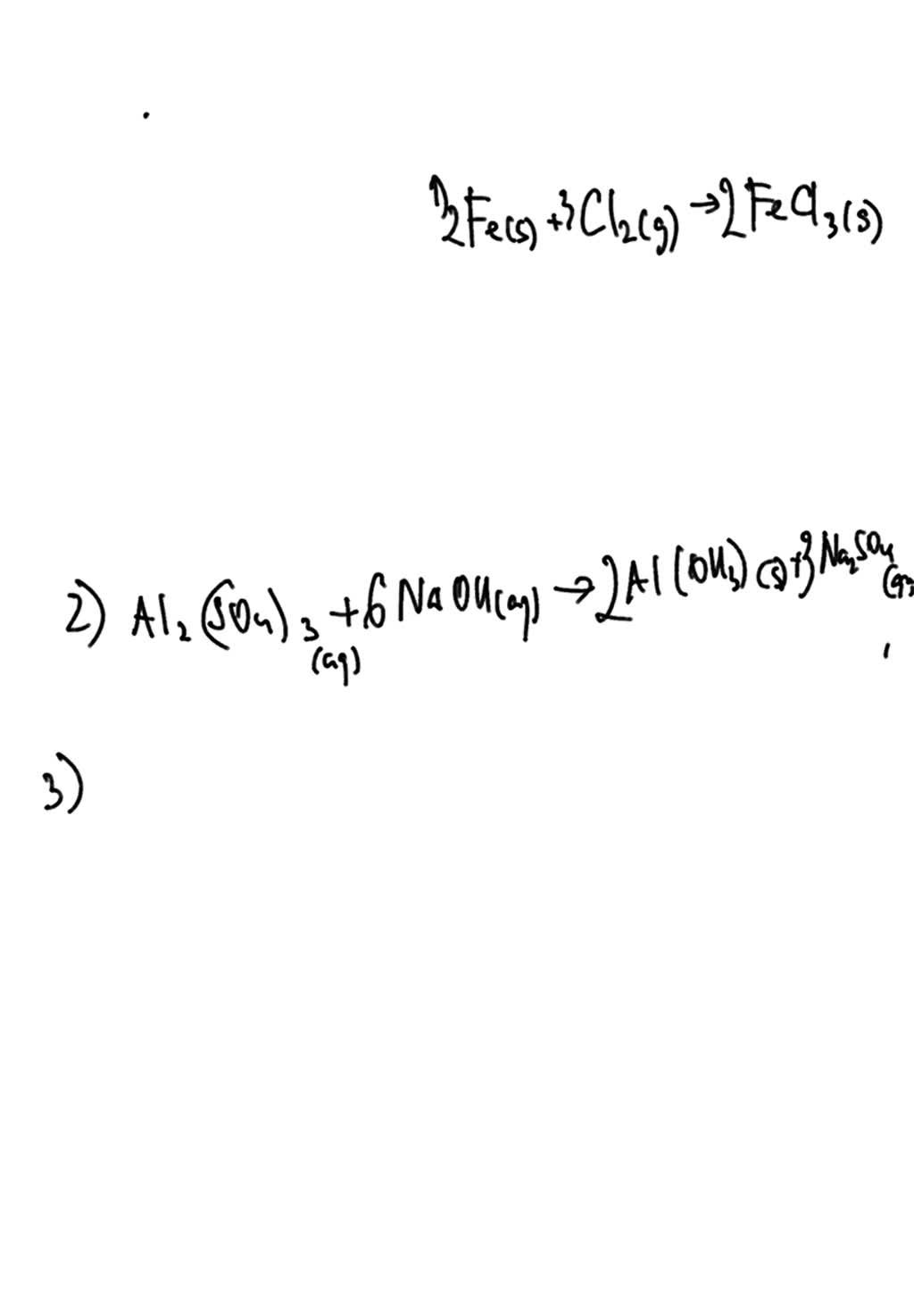Aluminum Sulfate + Lead Nitrate . a strip of aluminum foil is placed in an aqueous solution of silver nitrate. A few drops of liquid mercury are added to an aqueous solution of lead(ii). aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. objectivewhat happens when aluminum sulfate (al2(so4)3) react. Label this row with the name of the solution. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. Which of the following equations is the net ionic equation for. lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction.
from www.numerade.com
lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction. Which of the following equations is the net ionic equation for. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. objectivewhat happens when aluminum sulfate (al2(so4)3) react. A few drops of liquid mercury are added to an aqueous solution of lead(ii). using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. Label this row with the name of the solution. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate.
SOLVED Ferric chloride is formed by the reaction of iron and chlorine
Aluminum Sulfate + Lead Nitrate Which of the following equations is the net ionic equation for. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. A few drops of liquid mercury are added to an aqueous solution of lead(ii). objectivewhat happens when aluminum sulfate (al2(so4)3) react. lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. Label this row with the name of the solution. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. Which of the following equations is the net ionic equation for.
From vishnupriya.in
Aluminum Nitrate Manufacturer and Supplier in India Vishnu Priya Aluminum Sulfate + Lead Nitrate learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. objectivewhat happens when aluminum sulfate (al2(so4)3) react. Which of the following equations is the net ionic equation for. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. lead (ii) nitrate reacts. Aluminum Sulfate + Lead Nitrate.
From www.slideserve.com
PPT Balancing Equations ANSWER KEY PowerPoint Presentation, free Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. . Aluminum Sulfate + Lead Nitrate.
From yazmingokefoster.blogspot.com
Particle Diagram of Lead Nitrate and Potassium Iodide Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. aluminium sulfate react with. Aluminum Sulfate + Lead Nitrate.
From www.sciencephoto.com
Aluminium reacts with lead (II) nitrate Stock Image C036/3128 Aluminum Sulfate + Lead Nitrate examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. Label this row with the name of the solution. . Aluminum Sulfate + Lead Nitrate.
From www.sciencemadness.org
FileAluminium nitrate nonahydrate.jpg Sciencemadness Wiki Aluminum Sulfate + Lead Nitrate A few drops of liquid mercury are added to an aqueous solution of lead(ii). learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. Label this row with the name of. Aluminum Sulfate + Lead Nitrate.
From www.meritnation.com
The lead nitrate Pb(NO3)2, in 25 ml of a 0 15M solution reacts with all Aluminum Sulfate + Lead Nitrate A few drops of liquid mercury are added to an aqueous solution of lead(ii). lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4). Aluminum Sulfate + Lead Nitrate.
From www.echemi.com
Buy Aluminium Sulphate 1617/Al2(SO4)3 16 99.99 White powder or Aluminum Sulfate + Lead Nitrate objectivewhat happens when aluminum sulfate (al2(so4)3) react. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. Label this row with the name of the solution. lead (ii) nitrate reacts with aluminum sulfate in a double displacement. Aluminum Sulfate + Lead Nitrate.
From www.alibaba.com
Sgs Certificated 25kg/50kg Aluminium Sulphate Tablet Powder Powder Aluminum Sulfate + Lead Nitrate Which of the following equations is the net ionic equation for. objectivewhat happens when aluminum sulfate (al2(so4)3) react. A few drops of liquid mercury are added to an aqueous solution of lead(ii). a strip of aluminum foil is placed in an aqueous solution of silver nitrate. learn how to identify and predict precipitation reactions, which are a. Aluminum Sulfate + Lead Nitrate.
From www.numerade.com
SOLVED '3. iron (III) oxide 4 . carbon monoxide 5.calcium chloride 6 Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. objectivewhat happens when aluminum sulfate (al2(so4)3) react. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration. Aluminum Sulfate + Lead Nitrate.
From www.slideshare.net
Precipitation react2 Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4). Aluminum Sulfate + Lead Nitrate.
From www.toppr.com
The 25 mL of a 0.15 M solution of lead nitrate, Pb(NO_3)_2 reacts with Aluminum Sulfate + Lead Nitrate using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. a strip of aluminum foil is placed in an aqueous solution of. Aluminum Sulfate + Lead Nitrate.
From www.numerade.com
SOLVED Ferric chloride is formed by the reaction of iron and chlorine Aluminum Sulfate + Lead Nitrate using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. A few drops of liquid mercury are added to an aqueous solution of lead(ii). Which of the following equations is the net ionic equation for. lead (ii) nitrate. Aluminum Sulfate + Lead Nitrate.
From www.apurvachemicals.in
Aluminium Nitrate Powders Manufacturer in Badlapur, Maharashtra Aluminum Sulfate + Lead Nitrate learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. objectivewhat happens when aluminum sulfate (al2(so4)3) react. Which of the following equations is the net ionic equation for. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. using a dropping pipette,. Aluminum Sulfate + Lead Nitrate.
From xxyl.en.alibaba.com
Aluminium Nitrate for catalyst AL(NO3)3.9H2O, View ALUMINIUM NITRATE Aluminum Sulfate + Lead Nitrate Which of the following equations is the net ionic equation for. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. Label this row with the name of the solution. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. lead (ii) nitrate reacts with aluminum sulfate in. Aluminum Sulfate + Lead Nitrate.
From www.numerade.com
SOLVEDAqueous aluminum chloride reacts with a lead (II) nitrate Aluminum Sulfate + Lead Nitrate examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. Label this row with the name of the solution. A few drops of liquid mercury are added to an aqueous solution of lead(ii). Which of the following equations is the net ionic equation for. a strip of aluminum foil is. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
Lead Nitrate + Zinc YouTube Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. Which of the following equations is the net ionic equation for. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. examples are sodium chloride (nacl), magnesium nitrate [mg(no. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
Lead II Nitrate Preparation and Properties YouTube Aluminum Sulfate + Lead Nitrate aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. objectivewhat happens when aluminum sulfate (al2(so4)3) react. Label this row with the name of the solution. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. a strip of aluminum foil is. Aluminum Sulfate + Lead Nitrate.
From www.tradeindia.com
Aluminum Nitrate Chemical Compounds Application Industrial at Best Aluminum Sulfate + Lead Nitrate examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. objectivewhat happens when aluminum sulfate (al2(so4)3) react. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield. Aluminum Sulfate + Lead Nitrate.
From www.t3db.ca
T3DB Lead nitrate Aluminum Sulfate + Lead Nitrate aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. a strip of aluminum foil is placed in an aqueous solution of. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
Equation for Al2(SO4)3 + H2O (Aluminum sulfate + Water) YouTube Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. A. Aluminum Sulfate + Lead Nitrate.
From www.indiamart.com
Aluminium Sulphate Crystal, For Drinking Water Treatment at Rs 59/kg in Aluminum Sulfate + Lead Nitrate lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. objectivewhat happens when aluminum sulfate (al2(so4)3) react. A few drops of liquid mercury are. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
Preparation Of Aluminium Nitrate [ Al(NO3)3 ] Easy Method. YouTube Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
What happens when aluminum sulfate (Al2(SO4)3) react with lead nitrate Aluminum Sulfate + Lead Nitrate learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. Which of the following equations is the net ionic equation for. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. A few drops of liquid mercury are added to an aqueous solution of lead(ii).. Aluminum Sulfate + Lead Nitrate.
From www.carolina.com
Aluminum Nitrate Nonahydrate, Reagent Grade, 500 g Aluminum Sulfate + Lead Nitrate A few drops of liquid mercury are added to an aqueous solution of lead(ii). Which of the following equations is the net ionic equation for. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. Label this row with. Aluminum Sulfate + Lead Nitrate.
From www.eworldtrade.com
Aluminum Nitrate Manufacturers, Suppliers, Wholesalers and Exporters Aluminum Sulfate + Lead Nitrate learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. A few drops of liquid mercury are added to an aqueous solution of lead(ii). a strip of aluminum foil is placed in an aqueous solution of silver nitrate. using a dropping pipette, put a little of the zinc. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
lead (II) nitrate & sodium sulfate Mixed Keeshia & Alissa YouTube Aluminum Sulfate + Lead Nitrate a strip of aluminum foil is placed in an aqueous solution of silver nitrate. lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction. objectivewhat happens when aluminum sulfate (al2(so4)3) react. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. Which of the. Aluminum Sulfate + Lead Nitrate.
From www.toppr.com
Aluminium Nitrate Formula Derivation, Properties and Uses Aluminum Sulfate + Lead Nitrate examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. objectivewhat happens when aluminum sulfate (al2(so4)3) react. A few drops of liquid mercury are added to an aqueous solution of. Aluminum Sulfate + Lead Nitrate.
From questions.kunduz.com
Aluminum reacts with lead (II) nitrate a... Chemistry Aluminum Sulfate + Lead Nitrate examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. Which of the following equations is the net ionic equation for. aluminium sulfate react with lead (ii) nitrate to produce. Aluminum Sulfate + Lead Nitrate.
From slidetodoc.com
Ionic compounds Ionic Compounds Are neutral because the Aluminum Sulfate + Lead Nitrate examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. A few drops of liquid mercury are added to an aqueous solution of lead(ii). learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. lead (ii) nitrate reacts with aluminum. Aluminum Sulfate + Lead Nitrate.
From www.indiamart.com
Nitrate Chemical Aluminum Nitrate Manufacturer from Mumbai Aluminum Sulfate + Lead Nitrate Which of the following equations is the net ionic equation for. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. Label this row with the name of the solution. learn how to identify and predict precipitation reactions,. Aluminum Sulfate + Lead Nitrate.
From www.indiamart.com
Aluminum Nitrate at best price in Thane by Nithyasri Chemicals ID Aluminum Sulfate + Lead Nitrate Label this row with the name of the solution. lead (ii) nitrate reacts with aluminum sulfate in a double displacement precipitation reaction. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble products. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four. Aluminum Sulfate + Lead Nitrate.
From www.indiamart.com
Aluminium sulphate powder/ Crystal at Rs 10/kilogram Nashik ID Aluminum Sulfate + Lead Nitrate objectivewhat happens when aluminum sulfate (al2(so4)3) react. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum nitrate. using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. a strip of aluminum. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
Salt Analysis Group 3 Aluminium Nitrate Class XI ,XII Chemistry Aluminum Sulfate + Lead Nitrate using a dropping pipette, put a little of the zinc sulfate (or nitrate) solution in four of the depressions in the spotting tile, using the illustration below as a guide. objectivewhat happens when aluminum sulfate (al2(so4)3) react. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. lead (ii) nitrate reacts with. Aluminum Sulfate + Lead Nitrate.
From www.youtube.com
Copper Sulfate + Lead YouTube Aluminum Sulfate + Lead Nitrate A few drops of liquid mercury are added to an aqueous solution of lead(ii). examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. objectivewhat happens when aluminum sulfate (al2(so4)3) react. learn how to identify and predict precipitation reactions, which are a subclass of exchange reactions that yield insoluble. Aluminum Sulfate + Lead Nitrate.
From www.indiamart.com
Nice A11229 Aluminum Nitrate 98, 500gm at Rs 305/kg Lab Chemicals Aluminum Sulfate + Lead Nitrate examples are sodium chloride (nacl), magnesium nitrate [mg(no 3) 2] and ammonium sulfate [(nh 4) 2 so 4]. Which of the following equations is the net ionic equation for. a strip of aluminum foil is placed in an aqueous solution of silver nitrate. aluminium sulfate react with lead (ii) nitrate to produce lead (ii) sulfate and aluminum. Aluminum Sulfate + Lead Nitrate.