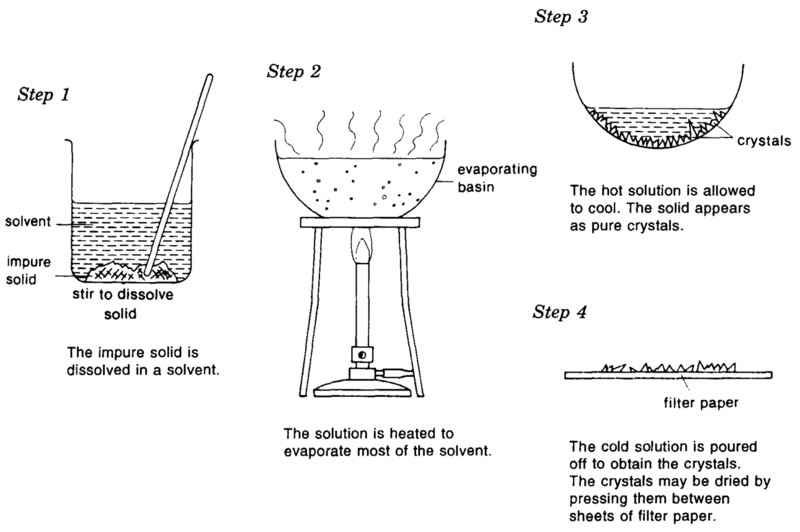
Filtration Distillation Crystallization Sublimation Chromatography . 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. chromatography involves solvent separation on a solid medium. Distillation (as discussed in analysis:. For a supersaturated solution, you can choose to let the solute crystallize out. Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. Rock candy (sugar solution) 4. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. fundamental experimental techniques of unitary operations: Distillation takes advantage of differences in boiling points. distillation, sublimation, and recrystallization use a change in physical state to effect a separation.
from byjus.com
Filtration separates solids of different sizes. Rock candy (sugar solution) 4. Evaporation removes a liquid from a solution to leave a solid material. Distillation (as discussed in analysis:. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. fundamental experimental techniques of unitary operations: chromatography involves solvent separation on a solid medium. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point.
Crystallization Definition, Process, Separation Technique, FAQs
Filtration Distillation Crystallization Sublimation Chromatography crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. fundamental experimental techniques of unitary operations: 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Distillation takes advantage of differences in boiling points. Rock candy (sugar solution) 4. Evaporation removes a liquid from a solution to leave a solid material. Distillation (as discussed in analysis:. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. chromatography involves solvent separation on a solid medium. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. For a supersaturated solution, you can choose to let the solute crystallize out. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. Filtration separates solids of different sizes.
From www.youtube.com
Separation of mixtures, sublimation, filtration, evaporation Filtration Distillation Crystallization Sublimation Chromatography Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). For a supersaturated solution, you can choose to let the solute crystallize out. for example, chromatography is a separation process based on solubility. Filtration Distillation Crystallization Sublimation Chromatography.
From www.youtube.com
How to separate Mixtures filtration, distillation, sublimation and Filtration Distillation Crystallization Sublimation Chromatography chromatography involves solvent separation on a solid medium. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. Filtration separates solids of different sizes. Rock candy (sugar solution) 4. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. For a supersaturated solution, you can choose to let the solute. Filtration Distillation Crystallization Sublimation Chromatography.
From slideplayer.com
MatterProperties and Changes ppt download Filtration Distillation Crystallization Sublimation Chromatography Distillation (as discussed in analysis:. fundamental experimental techniques of unitary operations: distillation, sublimation, and recrystallization use a change in physical state to effect a separation. Evaporation removes a liquid from a solution to leave a solid material. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. Rock candy (sugar. Filtration Distillation Crystallization Sublimation Chromatography.
From dokumen.tips
(PPT) 1. 2 The Different Separation Techniques are as follows Filtration Distillation Crystallization Sublimation Chromatography Filtration separates solids of different sizes. Distillation takes advantage of differences in boiling points. Rock candy (sugar solution) 4. fundamental experimental techniques of unitary operations: crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. Distillation (as. Filtration Distillation Crystallization Sublimation Chromatography.
From video.yckmc.edu.hk
Chemistry tutorialCh621Filtration, crystallization, simple Filtration Distillation Crystallization Sublimation Chromatography Filtration separates solids of different sizes. Distillation (as discussed in analysis:. chromatography involves solvent separation on a solid medium. For a supersaturated solution, you can choose to let the solute crystallize out. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. fundamental experimental techniques of unitary operations: Evaporation removes a liquid from. Filtration Distillation Crystallization Sublimation Chromatography.
From www.aakash.ac.in
Crystallization Process, Precautions, Advantages & Examples Filtration Distillation Crystallization Sublimation Chromatography fundamental experimental techniques of unitary operations: 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. for example, chromatography is a separation process based on solubility 1 and distillation is based on. Filtration Distillation Crystallization Sublimation Chromatography.
From www.youtube.com
Mixture Separation by Decantation, Filtration, Evaporation Filtration Distillation Crystallization Sublimation Chromatography 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). distillation, sublimation, and recrystallization use a change in physical state to effect a separation. For a supersaturated solution, you can choose to let the solute crystallize out. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and.. Filtration Distillation Crystallization Sublimation Chromatography.
From slideplayer.com
Matter Notes Note Slide 138 (Packet 1 ) , Slide (Packet 2) ppt download Filtration Distillation Crystallization Sublimation Chromatography crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). fundamental experimental techniques of unitary operations: distillation, sublimation, and recrystallization use a change in physical state to effect a separation. Rock candy. Filtration Distillation Crystallization Sublimation Chromatography.
From slideplayer.com
Chapter 2 Energy and Matter ppt download Filtration Distillation Crystallization Sublimation Chromatography evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. chromatography involves solvent separation on a solid medium. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. Distillation (as discussed in. Filtration Distillation Crystallization Sublimation Chromatography.
From slidetodoc.com
Basic Separation Techniques Filtration Crystallization Sedimentation Filtration Distillation Crystallization Sublimation Chromatography Distillation takes advantage of differences in boiling points. Evaporation removes a liquid from a solution to leave a solid material. For a supersaturated solution, you can choose to let the solute crystallize out. Filtration separates solids of different sizes. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. fundamental experimental. Filtration Distillation Crystallization Sublimation Chromatography.
From www.youtube.com
New 2 Chemistry GCSE Separating & Purifying Substances 1.2 Filtration Distillation Crystallization Sublimation Chromatography Evaporation removes a liquid from a solution to leave a solid material. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Rock candy (sugar solution) 4. Distillation takes advantage of differences in boiling. Filtration Distillation Crystallization Sublimation Chromatography.
From www.scribd.com
Separation Techniques Chromatography, Filtration, Distillation PDF Filtration Distillation Crystallization Sublimation Chromatography For a supersaturated solution, you can choose to let the solute crystallize out. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. . Filtration Distillation Crystallization Sublimation Chromatography.
From aimhigh.space
Distillation and Chromatography AimHigh! Filtration Distillation Crystallization Sublimation Chromatography chromatography involves solvent separation on a solid medium. fundamental experimental techniques of unitary operations: for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. crystallization is a favored technique to purify substances like. Filtration Distillation Crystallization Sublimation Chromatography.
From byjus.com
Crystallization Definition, Process, Separation Technique, FAQs Filtration Distillation Crystallization Sublimation Chromatography Filtration separates solids of different sizes. fundamental experimental techniques of unitary operations: crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. Evaporation removes a liquid from a solution to leave a. Filtration Distillation Crystallization Sublimation Chromatography.
From slideplayer.com
All About Matter SC2. Obtain, evaluate, and communicate information Filtration Distillation Crystallization Sublimation Chromatography Distillation (as discussed in analysis:. For a supersaturated solution, you can choose to let the solute crystallize out. Evaporation removes a liquid from a solution to leave a solid material. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Distillation takes advantage of differences in boiling points. fundamental experimental techniques. Filtration Distillation Crystallization Sublimation Chromatography.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Filtration Distillation Crystallization Sublimation Chromatography 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. For a supersaturated solution, you can choose to let the solute crystallize out. Filtration separates solids of different sizes. chromatography involves solvent separation. Filtration Distillation Crystallization Sublimation Chromatography.
From www.youtube.com
Science 9 Chapter 2 Evaporation sublimation, Chromatography Filtration Distillation Crystallization Sublimation Chromatography crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. Distillation takes advantage of differences in boiling points. Filtration separates solids of different sizes. Distillation (as discussed in analysis:. fundamental experimental techniques of unitary operations: For a supersaturated solution, you can choose to let the solute crystallize out. 2 this article. Filtration Distillation Crystallization Sublimation Chromatography.
From classnotes.org.in
Purification of Organic Compounds Chemistry, Class 11, Organic Filtration Distillation Crystallization Sublimation Chromatography chromatography involves solvent separation on a solid medium. Rock candy (sugar solution) 4. Distillation takes advantage of differences in boiling points. Distillation (as discussed in analysis:. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. For a supersaturated solution,. Filtration Distillation Crystallization Sublimation Chromatography.
From fyolagakf.blob.core.windows.net
Filtered Biology Definition at Mervin Moorhouse blog Filtration Distillation Crystallization Sublimation Chromatography Rock candy (sugar solution) 4. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Distillation takes advantage of differences in boiling points. Filtration separates solids of different sizes. distillation, sublimation, and. Filtration Distillation Crystallization Sublimation Chromatography.
From www.youtube.com
Chromatography Types, Retention Factor, Filtration, Distillation Filtration Distillation Crystallization Sublimation Chromatography evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. Rock candy (sugar solution) 4. Distillation (as discussed in analysis:. chromatography involves solvent separation on a solid medium. Distillation takes advantage of differences in boiling points. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. For. Filtration Distillation Crystallization Sublimation Chromatography.
From www.youtube.com
Separating techniques sublimation, chromatography and distillation Filtration Distillation Crystallization Sublimation Chromatography Evaporation removes a liquid from a solution to leave a solid material. chromatography involves solvent separation on a solid medium. Distillation (as discussed in analysis:. Filtration separates solids of different sizes. Rock candy (sugar solution) 4. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). For a supersaturated solution, you. Filtration Distillation Crystallization Sublimation Chromatography.
From www.slideserve.com
PPT Methods of Separating Mixtures PowerPoint Presentation, free Filtration Distillation Crystallization Sublimation Chromatography Filtration separates solids of different sizes. Evaporation removes a liquid from a solution to leave a solid material. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. Distillation takes advantage of differences in boiling points. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. Distillation (as. Filtration Distillation Crystallization Sublimation Chromatography.
From www.chemicals.co.uk
GCSE Chemistry Water Purification Guide The Chemistry Blog Filtration Distillation Crystallization Sublimation Chromatography fundamental experimental techniques of unitary operations: distillation, sublimation, and recrystallization use a change in physical state to effect a separation. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. chromatography involves solvent separation on a solid medium. Filtration separates solids of different sizes. crystallization is a favored technique to purify substances. Filtration Distillation Crystallization Sublimation Chromatography.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds Filtration Distillation Crystallization Sublimation Chromatography fundamental experimental techniques of unitary operations: For a supersaturated solution, you can choose to let the solute crystallize out. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. Distillation (as discussed in analysis:. distillation, sublimation,. Filtration Distillation Crystallization Sublimation Chromatography.
From byjus.com
Crystallization Definition, Process, Separation Technique, FAQs Filtration Distillation Crystallization Sublimation Chromatography crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. chromatography involves solvent separation on a solid medium. Rock candy (sugar solution) 4. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. Distillation (as discussed in analysis:. Filtration separates solids of. Filtration Distillation Crystallization Sublimation Chromatography.
From slideplayer.com
Chemistry. ppt download Filtration Distillation Crystallization Sublimation Chromatography crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). chromatography involves solvent separation on a solid medium. fundamental experimental techniques of unitary operations: evaporation procedures have led to useful separation. Filtration Distillation Crystallization Sublimation Chromatography.
From www.slideserve.com
PPT CHEMISTRY I PowerPoint Presentation, free download ID3104697 Filtration Distillation Crystallization Sublimation Chromatography Evaporation removes a liquid from a solution to leave a solid material. Distillation (as discussed in analysis:. chromatography involves solvent separation on a solid medium. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents. Filtration Distillation Crystallization Sublimation Chromatography.
From chem.libretexts.org
7.6 Classifying Separation Techniques Chemistry LibreTexts Filtration Distillation Crystallization Sublimation Chromatography Distillation (as discussed in analysis:. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. Evaporation removes a liquid from a solution to leave a solid material. Distillation takes advantage of differences in boiling points. Rock candy (sugar solution) 4. chromatography involves solvent separation on a solid medium. Filtration separates solids of different sizes. . Filtration Distillation Crystallization Sublimation Chromatography.
From www.slideserve.com
PPT Distillation & Chromatography PowerPoint Presentation, free Filtration Distillation Crystallization Sublimation Chromatography For a supersaturated solution, you can choose to let the solute crystallize out. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. fundamental experimental techniques of unitary operations: Filtration separates solids of different sizes. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. distillation,. Filtration Distillation Crystallization Sublimation Chromatography.
From slideplayer.com
Matter And Energy. ppt download Filtration Distillation Crystallization Sublimation Chromatography distillation, sublimation, and recrystallization use a change in physical state to effect a separation. Distillation (as discussed in analysis:. For a supersaturated solution, you can choose to let the solute crystallize out. Rock candy (sugar solution) 4. evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. for example, chromatography is a separation process. Filtration Distillation Crystallization Sublimation Chromatography.
From 12014dorcascharityng.weebly.com
FILTRATION,EVAPORATION,DISTILLATION AND PAPER CHROMATOGRAPHY 3E1 Filtration Distillation Crystallization Sublimation Chromatography Evaporation removes a liquid from a solution to leave a solid material. fundamental experimental techniques of unitary operations: 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). Filtration separates solids of different sizes. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. Distillation (as. Filtration Distillation Crystallization Sublimation Chromatography.
From collegedunia.com
Crystallization Definition, Process, Types & Examples Filtration Distillation Crystallization Sublimation Chromatography For a supersaturated solution, you can choose to let the solute crystallize out. distillation, sublimation, and recrystallization use a change in physical state to effect a separation. for example, chromatography is a separation process based on solubility 1 and distillation is based on boiling point. 2 this article looks at separation by particle size (filtration) and removal of. Filtration Distillation Crystallization Sublimation Chromatography.
From www.youtube.com
Organic Chemistry Class 11 Chemistry Chapter 2 Sublimation Filtration Distillation Crystallization Sublimation Chromatography chromatography involves solvent separation on a solid medium. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. Filtration separates solids of different sizes. For a supersaturated solution, you can choose to let the solute crystallize out. for example, chromatography is a separation process based on solubility 1 and distillation. Filtration Distillation Crystallization Sublimation Chromatography.
From www.pinterest.co.uk
Separation techniques filtration, distillation, chromatography Filtration Distillation Crystallization Sublimation Chromatography evaporation procedures have led to useful separation procedures, such as precipitation, crystallization, and. fundamental experimental techniques of unitary operations: chromatography involves solvent separation on a solid medium. Distillation (as discussed in analysis:. 2 this article looks at separation by particle size (filtration) and removal of volatile solvents (evaporation and crystallisation). for example, chromatography is a separation. Filtration Distillation Crystallization Sublimation Chromatography.
From slidetodoc.com
Purification of Materials Roman Boiko National University of Filtration Distillation Crystallization Sublimation Chromatography Evaporation removes a liquid from a solution to leave a solid material. crystallization is a favored technique to purify substances like sugar that decompose or may get charred when. Rock candy (sugar solution) 4. Distillation (as discussed in analysis:. Distillation takes advantage of differences in boiling points. distillation, sublimation, and recrystallization use a change in physical state to. Filtration Distillation Crystallization Sublimation Chromatography.