Lead + Sodium Hydroxide Balanced Equation . When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Precipitation of lead (ii) chloride. The balanced equation will be calculated along. Aqueous solutions of lead (ii) nitrate and sodium chloride. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. How do you balance chemical equations step by step? Let us use a double displacement reaction of lead (ii) nitrate. Balance any equation or reaction using this chemical equation balancer! Enter an equation of an ionic chemical equation and press the balance button. Find out what type of reaction occured.
from www.chegg.com
The balanced equation will be calculated along. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Precipitation of lead (ii) chloride. Enter an equation of an ionic chemical equation and press the balance button. Let us use a double displacement reaction of lead (ii) nitrate. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Balance any equation or reaction using this chemical equation balancer! How do you balance chemical equations step by step? Find out what type of reaction occured. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”.
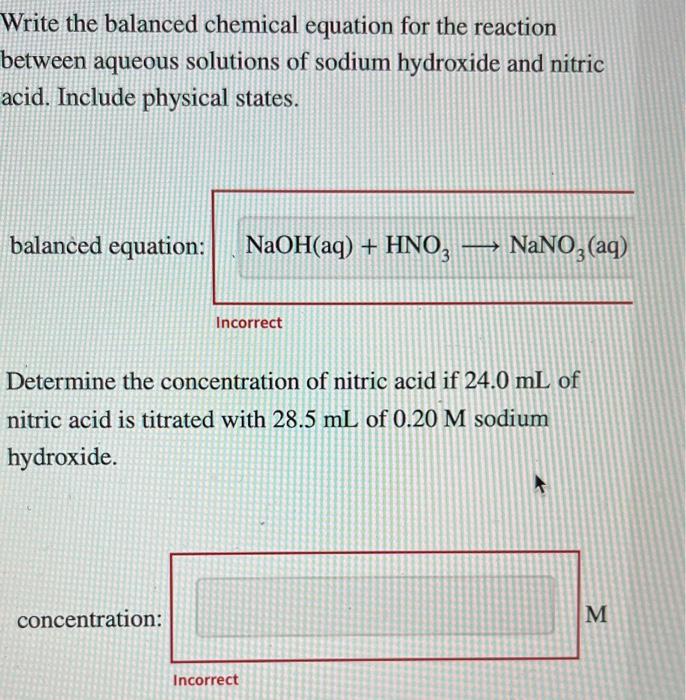
Solved Write the balanced chemical equation for the reaction
Lead + Sodium Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Precipitation of lead (ii) chloride. Let us use a double displacement reaction of lead (ii) nitrate. The balanced equation will be calculated along. Find out what type of reaction occured. Aqueous solutions of lead (ii) nitrate and sodium chloride. Balance any equation or reaction using this chemical equation balancer! Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. How do you balance chemical equations step by step? Enter an equation of an ionic chemical equation and press the balance button.
From www.chegg.com
Write the balanced chemical equation for each of the Lead + Sodium Hydroxide Balanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. How do you balance chemical equations step by step? Balance any equation or reaction using this chemical equation balancer! Precipitation of lead (ii) chloride. Find out what type of reaction occured. Enter an equation of an ionic chemical equation and press the balance button. The. Lead + Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced molecular equation for a reaction between Lead + Sodium Hydroxide Balanced Equation The balanced equation will be calculated along. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Aqueous solutions of lead (ii) nitrate and sodium chloride. Find out what type of reaction occured. Let us use a double displacement reaction of lead (ii) nitrate. Enter an equation of an. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Consider the chemical reaction that takes place Lead + Sodium Hydroxide Balanced Equation Aqueous solutions of lead (ii) nitrate and sodium chloride. How do you balance chemical equations step by step? When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Precipitation of lead (ii) chloride. Let us use a double displacement reaction of lead (ii) nitrate. Balance any equation or. Lead + Sodium Hydroxide Balanced Equation.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Lead + Sodium Hydroxide Balanced Equation How do you balance chemical equations step by step? When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. The balanced equation will be calculated along. Precipitation of lead (ii) chloride. Balance any. Lead + Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Lead (II) nitrate + potassium iodide Balanced Chemical Equation Lead + Sodium Hydroxide Balanced Equation Precipitation of lead (ii) chloride. Let us use a double displacement reaction of lead (ii) nitrate. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved a Balance the reaction of sodium hydroxide and Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! The balanced equation will be calculated along. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Aqueous solutions of lead (ii) nitrate and sodium chloride. Let us use a double displacement reaction of lead (ii) nitrate. Enter an. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved An aqueous solution of lead(II) nitrate is mixed with Lead + Sodium Hydroxide Balanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Balance any equation or reaction using this chemical equation balancer! How do you balance chemical equations step by step? Aqueous solutions of lead (ii) nitrate and sodium chloride. Let us use a double displacement reaction of lead (ii) nitrate. When carbon dioxide is dissolved in. Lead + Sodium Hydroxide Balanced Equation.
From www.meritnation.com
What is the coefficient of sodium hydroxide in a balanced chemical Lead + Sodium Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Find out what type of reaction occured. How do you balance chemical equations step by step? When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Balance any equation or reaction using this chemical equation. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Write the balanced chemical equation for each of the Lead + Sodium Hydroxide Balanced Equation Find out what type of reaction occured. How do you balance chemical equations step by step? Balance any equation or reaction using this chemical equation balancer! The balanced equation will be calculated along. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide,. Lead + Sodium Hydroxide Balanced Equation.
From kunduz.com
[ANSWERED] Write the balanced chemical equation for each of the Lead + Sodium Hydroxide Balanced Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Let us use a double displacement reaction of lead (ii) nitrate. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Diatomic chlorine and sodium hydroxide (lye). Lead + Sodium Hydroxide Balanced Equation.
From mariabsmitho.blob.core.windows.net
Lead Hydroxide Reaction Equation at mariabsmitho blog Lead + Sodium Hydroxide Balanced Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Precipitation of lead (ii) chloride. Find out what type of reaction occured. Enter an equation of an ionic chemical equation and press the balance button. Let us use a double displacement reaction of lead (ii) nitrate. The balancing. Lead + Sodium Hydroxide Balanced Equation.
From www.myxxgirl.com
What Is The Reaction Between Sodium Hydroxide And My XXX Hot Girl Lead + Sodium Hydroxide Balanced Equation Let us use a double displacement reaction of lead (ii) nitrate. How do you balance chemical equations step by step? Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enter an equation. Lead + Sodium Hydroxide Balanced Equation.
From www.cloudizsexy.com
What Is The Net Ionic Equation Slideshare Free Hot Nude Porn Pic Gallery Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! How do you balance chemical equations step by step? Find out what type of reaction occured. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Precipitation of lead (ii) chloride. Aqueous solutions of lead (ii) nitrate and sodium. Lead + Sodium Hydroxide Balanced Equation.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! The balanced equation will be calculated along. Let us use a double displacement reaction of lead (ii) nitrate. Find out what type of reaction occured. Precipitation of lead (ii) chloride. How do you balance chemical equations step by step? Aqueous solutions of lead (ii) nitrate and sodium chloride. Enter an. Lead + Sodium Hydroxide Balanced Equation.
From slsi.lk
slsi.lk how long for sulfatrim to work Touching phrase calcium Lead + Sodium Hydroxide Balanced Equation Enter an equation of an ionic chemical equation and press the balance button. Aqueous solutions of lead (ii) nitrate and sodium chloride. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Let us use a double displacement reaction of lead (ii) nitrate. How do you balance chemical equations step by step? Precipitation of lead. Lead + Sodium Hydroxide Balanced Equation.
From www.micoope.com.gt
Mol NaOH Reacts With Mol H3PO4 According To The Equation, 57 OFF Lead + Sodium Hydroxide Balanced Equation Aqueous solutions of lead (ii) nitrate and sodium chloride. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. The balanced equation will be calculated along. How do you balance chemical equations step by step? Find out what type of reaction occured. The balancing of a chemical equation is essential or necessary to fulfill the. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Write the balanced chemical equation for the reaction Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Enter an equation of an ionic chemical equation and press the balance button. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. How do you balance chemical equations step by step? Let us use a double displacement. Lead + Sodium Hydroxide Balanced Equation.
From iqclasses.in
Class 10 ICSE Chemistry Board Question Chapter Ammonia Lead + Sodium Hydroxide Balanced Equation Aqueous solutions of lead (ii) nitrate and sodium chloride. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balanced equation will be calculated along. How do you balance chemical. Lead + Sodium Hydroxide Balanced Equation.
From www.youtube.com
Write the balanced chemical equation for the reaction of sodium Lead + Sodium Hydroxide Balanced Equation Let us use a double displacement reaction of lead (ii) nitrate. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Balance any equation or reaction using this chemical equation balancer! The balancing. Lead + Sodium Hydroxide Balanced Equation.
From www.tessshebaylo.com
Balanced Equation For Reaction Of Ammonium Hydroxide With Hydrochloric Lead + Sodium Hydroxide Balanced Equation Aqueous solutions of lead (ii) nitrate and sodium chloride. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Let us use a double displacement reaction of lead (ii) nitrate. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. The balanced equation will be calculated along. The. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Lead (II) nitrate reacts with sodium iodide to Lead + Sodium Hydroxide Balanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Aqueous solutions of lead (ii) nitrate and sodium chloride. How do you balance chemical equations step by step? Precipitation of lead (ii) chloride. Enter an equation of an. Lead + Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDand net ionic equation for the following eactions Write Lead + Sodium Hydroxide Balanced Equation How do you balance chemical equations step by step? Let us use a double displacement reaction of lead (ii) nitrate. Balance any equation or reaction using this chemical equation balancer! The balanced equation will be calculated along. Enter an equation of an ionic chemical equation and press the balance button. Precipitation of lead (ii) chloride. Diatomic chlorine and sodium hydroxide. Lead + Sodium Hydroxide Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cr 3+ and NaOH YouTube Lead + Sodium Hydroxide Balanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Find out what type of reaction occured. Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer! The balancing of a chemical equation is essential or necessary to fulfill the requirement of. Lead + Sodium Hydroxide Balanced Equation.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Lead + Sodium Hydroxide Balanced Equation How do you balance chemical equations step by step? Enter an equation of an ionic chemical equation and press the balance button. Balance any equation or reaction using this chemical equation balancer! Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Aqueous solutions of lead (ii) nitrate and sodium chloride. Find out what type. Lead + Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVEDlead(Il) nitrate (aq) potassium iodide (a9) Observacion Lead + Sodium Hydroxide Balanced Equation Let us use a double displacement reaction of lead (ii) nitrate. How do you balance chemical equations step by step? Aqueous solutions of lead (ii) nitrate and sodium chloride. Enter an equation of an ionic chemical equation and press the balance button. Precipitation of lead (ii) chloride. The balanced equation will be calculated along. The balancing of a chemical equation. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved 5 Lead(11) Nitrate + Sodium Hydroxide Balanced Lead + Sodium Hydroxide Balanced Equation The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Aqueous solutions of lead (ii) nitrate and sodium chloride. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Enter an equation of an ionic chemical equation and press the balance button. Precipitation of. Lead + Sodium Hydroxide Balanced Equation.
From exohsvhug.blob.core.windows.net
Lead Oxide And Sodium Hydroxide Equation at Sebastian Malone blog Lead + Sodium Hydroxide Balanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Aqueous solutions of lead (ii) nitrate and sodium chloride. Find out what type of reaction occured. How do you balance chemical equations step by. Lead + Sodium Hydroxide Balanced Equation.
From www.youtube.com
Double displacement Pb(NO3)2 + NaOH Lead(II) nitrate + Sodium Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! How do you balance chemical equations step by step? Let us use a double displacement reaction of lead (ii) nitrate. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Precipitation of lead (ii) chloride. Find out what type of reaction occured. The balanced equation. Lead + Sodium Hydroxide Balanced Equation.
From signalticket9.pythonanywhere.com
Great Ammonia Plus Sulphuric Acid Balancing Equations Practice Problems Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. How do you balance chemical equations step by step? Precipitation of lead (ii) chloride. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with.. Lead + Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead + Sodium Hydroxide Balanced Equation Let us use a double displacement reaction of lead (ii) nitrate. Precipitation of lead (ii) chloride. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Balance any equation or reaction using this. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Write the balanced chemical equation for each of Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! Aqueous solutions of lead (ii) nitrate and sodium chloride. How do you balance chemical equations step by step? Find out what type of reaction occured. Let us use a double displacement reaction of lead (ii) nitrate. Precipitation of lead (ii) chloride. The balancing of a chemical equation is essential or. Lead + Sodium Hydroxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Lead + Sodium Hydroxide Balanced Equation The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Precipitation of lead (ii) chloride. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved Write The Balanced Chemical Equation For Each Of T... Lead + Sodium Hydroxide Balanced Equation The balanced equation will be calculated along. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Let us use a double displacement reaction of lead (ii) nitrate. Balance any equation or reaction using this chemical equation balancer! The balancing of a chemical equation is essential or necessary. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Solved 5. Lead(II) nitrate + sodium hydroxide → Balanced Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! Aqueous solutions of lead (ii) nitrate and sodium chloride. Precipitation of lead (ii) chloride. Find out what type of reaction occured. The balanced equation will be calculated along. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Enter an equation of an ionic chemical. Lead + Sodium Hydroxide Balanced Equation.
From www.chegg.com
Write A Balanced Chemical Equation For Each Of The... Lead + Sodium Hydroxide Balanced Equation Balance any equation or reaction using this chemical equation balancer! The balancing of a chemical equation is essential or necessary to fulfill the requirement of the “law of conservation of mass”. Aqueous solutions of lead (ii) nitrate and sodium chloride. The balanced equation will be calculated along. Precipitation of lead (ii) chloride. When carbon dioxide is dissolved in an aqueous. Lead + Sodium Hydroxide Balanced Equation.