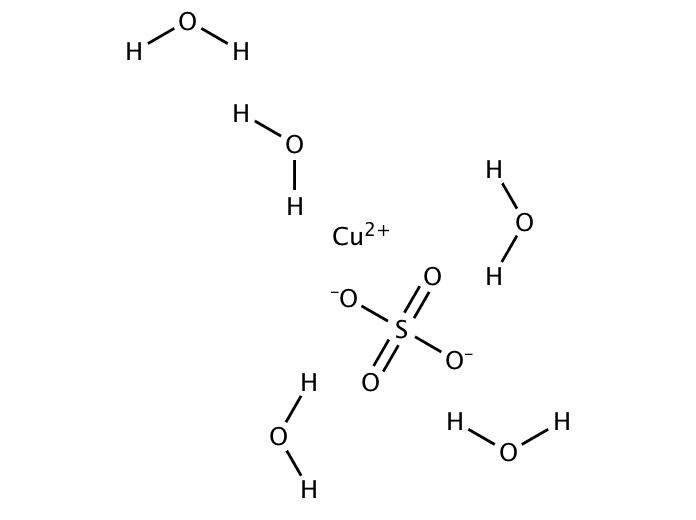
Copper Ii Sulfate Crystal Structure . the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. Its chemical formula is cuso. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. Melting point 200 °c with decomposition.
from school.careers360.com
the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. Melting point 200 °c with decomposition. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Its chemical formula is cuso.
cuso4 Overview, Structure, Properties & Uses
Copper Ii Sulfate Crystal Structure This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. Melting point 200 °c with decomposition. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. Its chemical formula is cuso. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o.
From www.pw.live
Copper Sulfate Formula, Structure, Properties, Uses Copper Ii Sulfate Crystal Structure the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four. Copper Ii Sulfate Crystal Structure.
From commons.wikimedia.org
FileCopper(II)sulfatepentahydrateCu1coordxtal2007CM3Dballs Copper Ii Sulfate Crystal Structure Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. Melting point 200 °c with decomposition. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline. Copper Ii Sulfate Crystal Structure.
From school.careers360.com
cuso4 Overview, Structure, Properties & Uses Copper Ii Sulfate Crystal Structure copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Melting point 200 °c with decomposition. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four. Copper Ii Sulfate Crystal Structure.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. the structure of copper (ii) sulfate, specifically in its. Copper Ii Sulfate Crystal Structure.
From ar.inspiredpencil.com
Aqueous Copper Sulfate Copper Ii Sulfate Crystal Structure the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. Melting point 200 °c with decomposition. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric. Copper Ii Sulfate Crystal Structure.
From www.fishersci.com
Copper (II) Sulfate, 1, Fisher Chemical Fisher Scientific Copper Ii Sulfate Crystal Structure Melting point 200 °c with decomposition. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. the systematic name for cuso 4 is copper(ii) sulfate, but it is. Copper Ii Sulfate Crystal Structure.
From crystallography365.wordpress.com
Copper sulfate Crystallography365 Copper Ii Sulfate Crystal Structure Its chemical formula is cuso. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Inorganic compound, salt of bivalent. Copper Ii Sulfate Crystal Structure.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. This arrangement allows the copper ion. Copper Ii Sulfate Crystal Structure.
From www.sciencephoto.com
Hydrated copper sulphate crystals Stock Image C010/9660 Science Copper Ii Sulfate Crystal Structure copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Its chemical formula is cuso. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. the crystal structure of copper sulphate pentahydrate, cuso. Copper Ii Sulfate Crystal Structure.
From www.researchgate.net
The crystal model of copper sulfide (covellite). Download Scientific Copper Ii Sulfate Crystal Structure Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. Its chemical formula is cuso. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1]. Copper Ii Sulfate Crystal Structure.
From ar.inspiredpencil.com
Copper Sulfate Crystals Copper Ii Sulfate Crystal Structure the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Its chemical formula is. Copper Ii Sulfate Crystal Structure.
From www.shutterstock.com
Copper (Ii) Sulfate (Cuso4, Cupric Sulfate), Crystal Structure. This Copper Ii Sulfate Crystal Structure Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical. Copper Ii Sulfate Crystal Structure.
From dxolgyxds.blob.core.windows.net
Copper Sulfate Large Crystals at Gary Land blog Copper Ii Sulfate Crystal Structure Its chemical formula is cuso. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Melting point 200 °c with decomposition. the structure of copper. Copper Ii Sulfate Crystal Structure.
From molekula.com
Purchase Copper(II) sulfate anhydrous [7758987] online • Catalog Copper Ii Sulfate Crystal Structure copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a. Copper Ii Sulfate Crystal Structure.
From www.pngwing.com
Copper(II) sulfate Crystal structure Chemical compound, salt, natural Copper Ii Sulfate Crystal Structure This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. Its chemical formula is cuso. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or. Copper Ii Sulfate Crystal Structure.
From crystalverse.com
The Best Way to Grow Big Copper Sulfate Crystals Crystalverse Copper Ii Sulfate Crystal Structure Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. Melting point 200 °c with decomposition. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. the structure of. Copper Ii Sulfate Crystal Structure.
From www.alamy.com
Copper(II) sulfate with molecular structure. Chemical ingredient used Copper Ii Sulfate Crystal Structure Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Melting point 200 °c with decomposition. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry.. Copper Ii Sulfate Crystal Structure.
From www.alamy.com
Copper (II) sulfate (CuSO4, cupric sulfate), crystal structure. Bright Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Its chemical formula is cuso. Melting point 200 °c with decomposition. copper(ii) sulfate, also known as cupric. Copper Ii Sulfate Crystal Structure.
From www.funcmater.com
wholesale Ammonium copper(II) sulfate hexahydrate Crystalline FUNCMATER Copper Ii Sulfate Crystal Structure Melting point 200 °c with decomposition. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Its chemical formula is cuso. This arrangement allows the copper ion to. Copper Ii Sulfate Crystal Structure.
From www.pngwing.com
Copper(II) nitrate Copper(II) sulfate Structure, oxygen, blue Copper Ii Sulfate Crystal Structure Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a. Copper Ii Sulfate Crystal Structure.
From imgbin.com
Copper(II) Sulfate Crystal Structure PNG, Clipart, Berry, Chemistry Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Its chemical formula is cuso. This arrangement allows the copper ion to interact with the sulfate ions, forming. Copper Ii Sulfate Crystal Structure.
From melscience.com
Copper(II) sulfate MEL Chemistry Copper Ii Sulfate Crystal Structure Melting point 200 °c with decomposition. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central. Copper Ii Sulfate Crystal Structure.
From exojucsti.blob.core.windows.net
Copper Sulfate Use In Ponds at John Fleming blog Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Melting point 200 °c with decomposition. Its chemical formula is cuso.. Copper Ii Sulfate Crystal Structure.
From ar.inspiredpencil.com
Copper Ii Sulfate Structure Copper Ii Sulfate Crystal Structure Its chemical formula is cuso. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Melting point 200 °c with decomposition.. Copper Ii Sulfate Crystal Structure.
From pnghero.com
Crystal Ball Copper(II) Sulfate Structure PNG Image PNGHERO Copper Ii Sulfate Crystal Structure Its chemical formula is cuso. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged. Copper Ii Sulfate Crystal Structure.
From ar.inspiredpencil.com
Copper Ii Sulfate Structure Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Its chemical formula is cuso. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Melting point 200 °c with decomposition. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. the systematic name for. Copper Ii Sulfate Crystal Structure.
From www.pngegg.com
Copper(II) sulfate Hydrate Copper(II) oxide Anhydrous, filling, natural Copper Ii Sulfate Crystal Structure Its chemical formula is cuso. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper,. Copper Ii Sulfate Crystal Structure.
From www.verywellhealth.com
Copper Sulfate Uses, Benefits, and Warnings Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. Its chemical formula is cuso. the systematic name for cuso 4 is copper(ii) sulfate, but. Copper Ii Sulfate Crystal Structure.
From www.thoughtco.com
How to Grow Blue Copper Sulfate Crystals Copper Ii Sulfate Crystal Structure copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four sulfate (so₄²⁻) ions arranged in a tetrahedral geometry. Its chemical formula is cuso. the systematic name for. Copper Ii Sulfate Crystal Structure.
From exospwven.blob.core.windows.net
Copper (Ii) Sulfate Is The And Water Is The at Ben Pratt blog Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Melting point 200 °c with decomposition. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features a central copper ion (cu²⁺) connected to four. Copper Ii Sulfate Crystal Structure.
From pnghut.com
Copper(II) Sulfate Crystal Structure Copperii Pentahydrate Ball Copper Ii Sulfate Crystal Structure the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Inorganic compound, salt of bivalent transitional metal copper and inorganic sulfuric acid. Melting point 200 °c with decomposition. Its chemical formula is cuso. copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the systematic name for. Copper Ii Sulfate Crystal Structure.
From imgbin.com
Copper(II) Sulfate Crystal Structure PNG, Clipart, Angle, Anhydrous Copper Ii Sulfate Crystal Structure copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. the structure of copper (ii) sulfate, specifically in its anhydrous form (cuso₄), features. Copper Ii Sulfate Crystal Structure.
From edu.svet.gob.gt
What Would A Diagram Of The Lattice Structure Of CuSO4 Look Copper Ii Sulfate Crystal Structure copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. Its chemical formula is cuso. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue. Copper Ii Sulfate Crystal Structure.
From www.reddit.com
Some Copper II Sulfate pentahydrate that I recrystallised from the Copper Ii Sulfate Crystal Structure copper(ii) sulfate, also known as cupric sulfate, copper sulfate, blue vitriol, [1] or bluestone, [1] is a chemical compound. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. the crystal structure of copper sulphate pentahydrate, cuso 4.5h 2 o. Its chemical. Copper Ii Sulfate Crystal Structure.
From www.wikidoc.org
Copper(II) sulfate wikidoc Copper Ii Sulfate Crystal Structure Its chemical formula is cuso. This arrangement allows the copper ion to interact with the sulfate ions, forming a solid crystalline structure. the systematic name for cuso 4 is copper(ii) sulfate, but it is also referred to as blue vitriol, roman vitriol, the vitriol of copper, and. Melting point 200 °c with decomposition. the structure of copper (ii). Copper Ii Sulfate Crystal Structure.